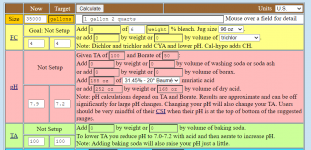
When lowering the pH from 8.0 to 7.2, the percentage of borate goes from 6.6% to 1.1%.
So, the buffering depends on how much borate needs to be converted into boric acid.
pH.....Borate.....Boric Acid.
7.2.....1.1%..........98.9%
7.4.....1.7%..........98.3%
7.6.....2.7%..........97.3%
7.8.....4.3...........95.7%
8.0.....6.6...........93.4%.
B(OH)
4- + H
+ --> B(OH)
3 + H
2O.
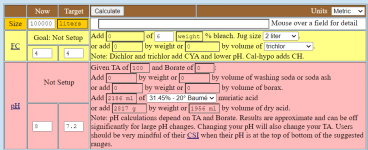
Every mole of borate that has to be converted from borate to boric acid requires 1 mole of hydrogen ions.
50 ppm boron in 100,000 liters is 5,000 grams of boron.
5,000 grams of boron is 462.4919 moles of boron or borate.
6.6% of 462.4919 is 30.52 moles.
1.1% of 462.4919 is 5.0874 moles.
So, we need to convert 25.43 moles of borate to boric acid, which will take 25.43 moles of hydrochloric acid.
25.43 moles of hydrogen chloride is 927.27 grams of hydrogen chloride.
20 baume contains 370.88 g HCl/liter.
927.27 kg/370.88 kg = 2.500 liters of HCl.
4.873 liters - 2.186 liters = 2.687 liters of 31.45% acid needed to convert the borate to boric acid.
__________________________________________________________________________________________________________
Total Acidity is the same type of concept of a buffer but in reverse.
For example, to change the pH from 7.2 to 8.0 with sodium hydroxide, we need to convert 25.43 moles of boric acid to borate, which will take 25.43 moles of sodium hydroxide, which is 1,017 grams of sodium hydroxide.
C
3H
2N
3O
3 + H
+ --> C
3H
3N
3O
3
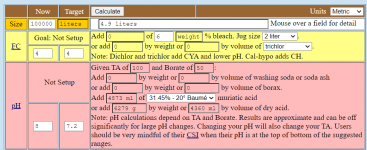
Every mole of cyanurate that has to be converted from cyanurate to cyanuric acid requires 1 mole of hydrogen ions.
50 ppm cyanuric acid in 100,000 liters is 5,000 grams of cyanuric acid.
5,000 grams of cyanuric acid is 38.74 moles of cyanuric acid.
The cyanurate percentage goes from 93 to 68%, which is 25%
25% of 38.74 moles is 9.685 moles.
So, we need to convert 9.685 moles of cyanurate to cyanuric acid, which will take 9.685 moles of hydrochloric acid.
9.685 moles of hydrogen chloride is 353.12 grams of hydrogen chloride.
20 baume contains 370.88 g HCl/liter.
353.12 g/370.88 g = 0.952 liters of HCl.
So, it takes 0.952 liters of 31.45% hydrochloric acid to convert the cyanurate to cyanuric acid.
You can do the same calculation for the carbonate and bicarbonate to see the amount of acid needed for that buffer.
The remaining amount of acid goes towards actually lowering the pH.
As you can see, the buffers really absorb a lot of the acid and the amount of acid that actually changes the pH is very small.