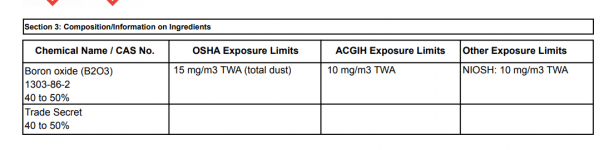
I'm a fairly new pool owner. I bought the Easy Pool Chemistry book, read it cover to cover, followed each step meticulously, and was doing amazingly well until I added my borate. I can't figure out what happened, and I'm so frustrated. I got my CYA up to 50 ppm, got my calcium hardness to 362 ppm, brought my alkalinity up to 94 ppm with a ph of 7.5, and everything was looking fantastic. I added two doses of borate about 8 hours apart to get it up to around 50 ppm (I think I probably overshot it, but not by much. Maybe it's at 60 or 70 now. Test strips aren't so precise.)
Anyway, I added some cal-hypo (because my chlorine was very low), let the pool run overnight, and now at 10:30 in the morning my pH and alkalinity are through the roof! pH went up to 8.0, and alkalinity is now testing at 219 ppm, and I know it's not a bad reading because I use the Lamotte digital reading with liquid drops, and the alkalinity drops that are supposed to be kind of grass-green turned the water sample dark blue!
I guess my question is, how did this happen, and is this just some temporary effect that will go back to normal once the borate gets thoroughly mixed in? For reference, I used Pro Team Supreme Plus, which is supposed to be mostly boric acid, and specifically says it's a pH neutral compound that won't effect your pH at all. What I'm really dumbfounded about is why I spent all that time meticulously adjusting my alkalinity to a target of 90 ppm only to have the borate knock it off the charts. Anybody know what happened here?
Anyway, I added some cal-hypo (because my chlorine was very low), let the pool run overnight, and now at 10:30 in the morning my pH and alkalinity are through the roof! pH went up to 8.0, and alkalinity is now testing at 219 ppm, and I know it's not a bad reading because I use the Lamotte digital reading with liquid drops, and the alkalinity drops that are supposed to be kind of grass-green turned the water sample dark blue!
I guess my question is, how did this happen, and is this just some temporary effect that will go back to normal once the borate gets thoroughly mixed in? For reference, I used Pro Team Supreme Plus, which is supposed to be mostly boric acid, and specifically says it's a pH neutral compound that won't effect your pH at all. What I'm really dumbfounded about is why I spent all that time meticulously adjusting my alkalinity to a target of 90 ppm only to have the borate knock it off the charts. Anybody know what happened here?