Let's start with a confession: I am a pool nerd.
I understand that the Taylor phenol red test is a reliable, easy to use pH test with a resolution that is absolutely sufficient for pool maintenance.
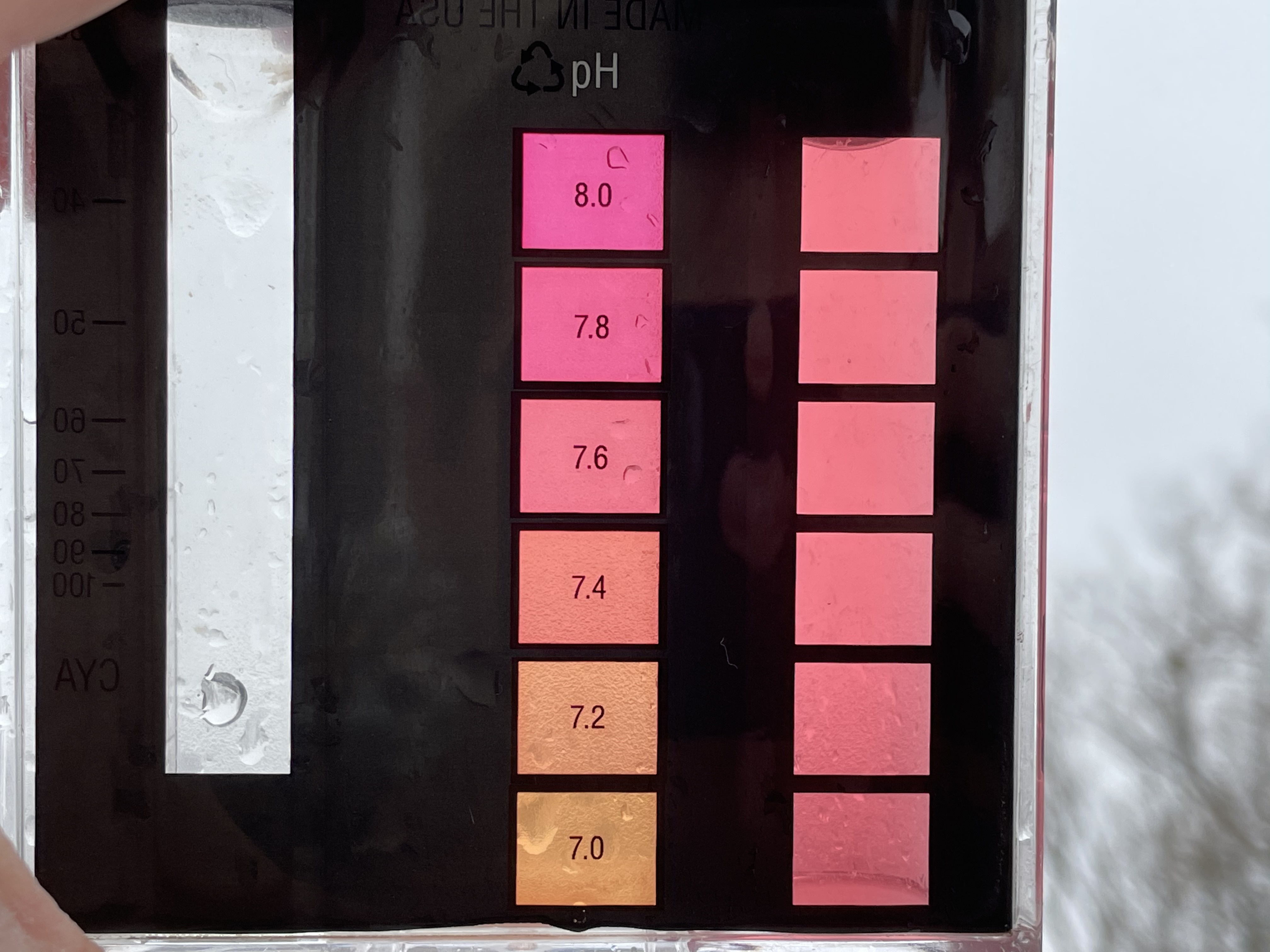
As long as one is able to differentiate the different shades of orange/red. For the vast majority of people, that seems to be the case, no need to reinvent the wheel or pay unnecessary money, just go with it. TFP's recommend and preferred option, no discussion about that.
But there seem to be at least a few around, that are struggling with that. And I was glad to get some reassurance on this forum that I am not the only one. And to learn about alternatives, and the limitations and downsides of these alternatives, and not just being told that there is something wrong with me, and I have to practice harder to be able to use the phenol test.
I think it is important for those using pH-meters, to understand that just because it is showing a number on a digital display, that this number isn't necessarily correct. It needs regular calibration. You have to understand that the integrated temperature compensation can only work if the integrated thermometer has sufficiently thermalized to the sample temperature. And when a continued slow drift of the reading should be ignored, and when the error can be in the range of 0.2 (as in the case of sticking a meter that's been stored in a heated room in cold pool water in winter).
And I am very grateful for
@AUSpool's contributions pointing out the influence of translucency, that there are other options to using a pH-meter when struggling with colour shades. And for his and
@jseyfert3's comments that it seems to be easier to differentiate between shades on photos than in real life - I was thinking the same, and that gives me hope that a comparator that mimics the translucency of real samples might do the trick for me.
Part of the discussion was also about the Clear Choice Labs comparator that the folks from Down Under are predominantly using, with a scale only up to 7.8 - which is a clear downside when pH settles in the 7.6 - 7.8 range.