Howdy,
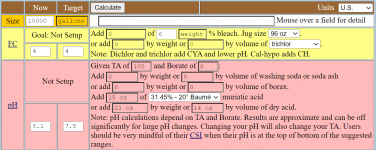
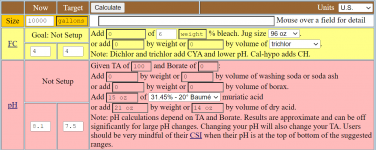
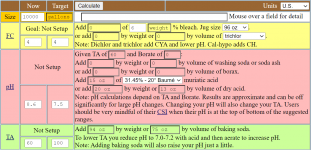
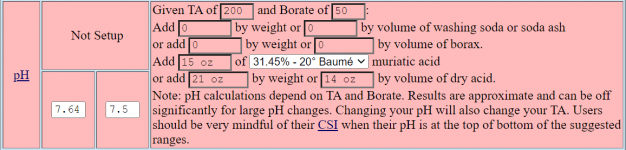
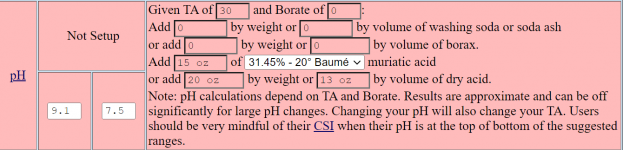
I use the Taylor K-2006 kit to test my water. I've always had trouble pinning down pH when it's in the purple range, meaning 7.6 and up pretty much look the same to me. But once I starting adding the R-0005 acid demand reagent, I can see when I hit 7.5.
I want to start using TFP's guidelines for keeping pH between 7.6 and 7.8... not inch up to 8.0 and beyond. So somehow I have to figure out how to calculate current pH using the number of drops of R-0005. I just can't figure it out.
So my question is: If the indicator test (R-0004) shows pH is "purple" and it takes just one drop to get it to 7.5, what was the beginning pH? And what if it takes 2 drops? (since I understand pH is logarithmic???)
Tx
I use the Taylor K-2006 kit to test my water. I've always had trouble pinning down pH when it's in the purple range, meaning 7.6 and up pretty much look the same to me. But once I starting adding the R-0005 acid demand reagent, I can see when I hit 7.5.
I want to start using TFP's guidelines for keeping pH between 7.6 and 7.8... not inch up to 8.0 and beyond. So somehow I have to figure out how to calculate current pH using the number of drops of R-0005. I just can't figure it out.
So my question is: If the indicator test (R-0004) shows pH is "purple" and it takes just one drop to get it to 7.5, what was the beginning pH? And what if it takes 2 drops? (since I understand pH is logarithmic???)
Tx