Dirk TFP has been a great find for you. You have been a great find for TFP! Thanks for sharing all you are doing and learning so others can learn with you. 
Kim

Kim



Dirk TFP has been a great find for you. You have been a great find for TFP! Thanks for sharing all you are doing and learning so others can learn with you.



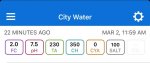
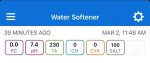
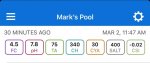
The water out of the softener will have varying levels of calcium that change with age and use of the resin...all of it will be well below what your test kit is capable of measuring. So it’s not really “0” in reality but, for practical purposes, you can consider it to be < 10ppm. That’s good enough for pool fill water.

I only use 4 drops in my K1000 pH test. It helps me compare colors. Try it.
I've been using this advice with great success. But...
I just had a conversation with Wayne from Taylor. I was asking him about the accuracy of their K2006 tests. In the course of our conversation, I asked him about this 4-drop idea. He was quite adamant that this was not a good idea, and insisted the accuracy of the pH test depends on 5 drops.
....
The call to Taylor was inspired by the CYA test result. It's unfortunate that a number that is so critical to proper FC (and pool) maintenance is so difficult to accurately obtain.



Taylor's warnings are appropriate because color interpretation is a very tricky thing. There are two things to understand about pH indicators -
1. The color, or hue, of the solution is set by the pH of solution and the pKa of indicator dye.
2. The saturation of the color (also known has the chroma or intensity) is determined by the concentration of that color in the solution
To point #1, the chemistry is simple acid/conjugate base reaction -
HIn = protonated Indicator molecule (yellow color)
H+ = acid proton in solution
In- = Negatively charge form of the indicator dye with proton removed (red color)
HIn(aq) <-----> H+ (aq) + In- (aq)
Ka = ([H+]*[In-]) / [HIn]
or, if we do some fancy math with logarithms, you eventually get the Henderson-Hasselbalch equation -
pH = pKa + log([In-]/[HIn])
So, the ratio of the deprotonated form of the phenol red indicator ([In-] which optically looks red colored) and the protonated form of the phenol red indicator ([HIn] which optically looks yellow) is strictly determined by the difference between the pH of the solution and the pKa of the chemical compound. So, the hue (which would be a mixture of red and yellow) is set entirely by the pH of the solution since the pKa is a constant. So the ratio never changes but, as the total concentration of those chemicals is reduced in solution, whatever the hue is, it's saturation will decrease towards white (colorless on a white background).
There is also the added complexity that you are comparing your solution color to a color standard sitting next to it. That color standard has a hue and saturation that is defined by the concentration of the dye in the medium. So, when Taylor makes their comparator block, they are trying to exactly match the hue and saturation of the indicators color at a given pH.
I did a test of 5 drops versus 4 drops side by side since I have two #9056 comparator tubes -
5 drops of R-0004
View attachment 73442
4 drops of R-0004
View attachment 73443
PS - The answer is 7.6 ....
I'm sure the forum software will downgrade the image resolution but, if you could see them on my phone, you might be tempted to think they are different colors. They are not. In the bright sun with my white background they are clearly the same color just a slightly different saturation or intensity due to the 20% lower concentration in solution. As I see them, the 5 drop sample looks closest to the 7.6 color patch on the tube.
I also have R-0014 drops and the midget comparator tube that's similar to what is found in the K-1000 or K-1001 kits. That comparator has a 7.5 pH color swatch on it and the color of the solution does not look like 7.5 but between 7.5 and 7.8 on that midget tube.

