- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
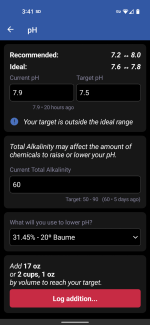
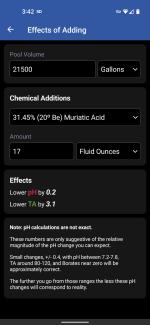
Here it is with CYA of 300 ... recommending 117oz
You can't enter parameter combinations into PoolMath that aren't chemically possible, PoolMath is not clever enough to realise that. If you enter TA 100, then changing CYA will not have an impact on the calculation, as PoolMath simply takes the TA value for the MA estimation.
As Marty already pointed out, with CYA 300, the CYA-Alkalinity alone would already be about 100. With CO2 being in equilibrium with atmospheric CO2, TA needed to be at least about 150 to be in equilibrium at pH 8.3. 117oz of MA would get you down to 7.8 in this scenario.