We sold a refurbished Mastertemp 400 to a customer a few weeks ago. It ran for 2-3 weeks without issue then the customer calls me last week to tell me it's leaking for the air intake and that the heat exchanger is damaged. I of course want to do right by the customer. I didn't visually inspect the exchanger myself, though my tech is telling me it was sound (I don't know if he did a visual inspection or not). Speaking with the customer, he is telling me measured his pH at "6.2ish" and corrected it. I'm going to stop by today and check the alkalinity and CH to see what the LSI is, but from experience, how long should a good condition exchanger withstand poor water condition?
How quickly can pH/negative LSI eat through a heat exchanger?
- Thread starter QPSUtah
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
It is very unlikely that they measured the pH at 6.2.Speaking with the customer, he is telling me measured his pH at "6.2ish" and corrected it.
The TA was probably zero from tabs use.
It depends on the pH and chlorine levels.
Most likely, the customer uses trichlor tabs and the tab feeder sent corrosive liquid into the heat exchanger while the pump was off.
Last edited:
Had a brand new one that I installed for another company fail exactly like that in less than six months, probably faster as he didn't tell me until he tried to find out what was wrong first. The customer re-installed the tablet feeder I removed before I would install the heater. I refuse to install a heater on a system that has a tab feeder. He ended up buying them a new heater that he installed and left the feeder.We sold a refurbished Mastertemp 400 to a customer a few weeks ago. It ran for 2-3 weeks without issue then the customer calls me last week to tell me it's leaking for the air intake and that the heat exchanger is damaged. I of course want to do right by the customer. I didn't visually inspect the exchanger myself, though my tech is telling me it was sound (I don't know if he did a visual inspection or not). Speaking with the customer, he is telling me measured his pH at "6.2ish" and corrected it. I'm going to stop by today and check the alkalinity and CH to see what the LSI is, but from experience, how long should a good condition exchanger withstand poor water condition?
Fiberglass pool, tab feeder, shutting the pump off each night = very low pH, almost zero alkalinity, destroyed heater.
You sometimes learn the hard (expensive) way, don't sell used heaters unless you have seen them in service first, then completely disassembled it to see the internals. Its just not worth it, they're too expensive to reinstall the parts with new O rings, sensors, etc. (not counting your labor), and to warranty.
The only way to tell if the exchanger on a MaterTemp/Max-E-Therm heater is good is to completely disassemble down to opening the tub and removing the exchanger, so no, no one did, or would do, a visual inspection
When customers would ask if there were used heaters for sale I would tell them that the only logical reason to remove a pool heater is because it isn't working or it has a problem, like a leak.
Last edited:
- Nov 23, 2014
- 213
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
Good advice and feedback, methinks. I'm just wondering how 'close inspection' and disassembly would reveal a heat exchanger that was about to leak. If a highly acidic environment were degrading the heat exchanger, wouldn't all that degradation be located inside the exchanger (which to my knowledge does not come apart)? Asking in case I ever buy another used heaterthen completely disassembled it to see the internals
- Jul 21, 2013
- 65,062
- Pool Size
- 35000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
You can judge the condition of the MasterTemp heat exchanger if you pull off the manifold and check the condition of the tubes that connect to the manifold. If they are worn then the exchanger is worn.Good advice and feedback, methinks. I'm just wondering how 'close inspection' and disassembly would reveal a heat exchanger that was about to leak. If a highly acidic environment were degrading the heat exchanger, wouldn't all that degradation be located inside the exchanger (which to my knowledge does not come apart)? Asking in case I ever buy another used heaterThanks.

Corrosive water will "eat" holes into the copper. The tubes will leak water into the sealed tub when the pump is running and eventually come out the blower. I've seen it happen too many times on those heaters when the water chemistry is very bad.Good advice and feedback, methinks. I'm just wondering how 'close inspection' and disassembly would reveal a heat exchanger that was about to leak. If a highly acidic environment were degrading the heat exchanger, wouldn't all that degradation be located inside the exchanger (which to my knowledge does not come apart)? Asking in case I ever buy another used heaterThanks.
If it happened at 2 to 3 weeks, an inspection of the exchanger would likely have shown up some "green" spots from the reaction of the water, air and copper, indicating a leak, even a very tiny one. Looking at the front end of the tubes after removing the manifold would have been a good indication, but doesn't tell you what is happening inside the tub.
Unlike selling a used car "as is," in California, as a contractor, I have to provide at least a one year warranty on anything I install. Not sure how that works in Florida. I couldn't make enough on a used heater to stand that kind of loss.
I did sell one. It came off a customer's pool. It had been in extremely light use for about three years after I installed it. The temperature sensor failed. He, quite literally having more money than he needed, decided that a $150.00 repair wasn't good enough and bought a new heater.
It went to a very nice family with a lot of kids that couldn't begin to afford new. They use it for the next 8 years then moved. It may be working still for all I know. And no, they didn't have a tablet feeder.
Last edited:
I’m here measuring chems. TA is 120 or so. CH is 1250, very high.It is very unlikely that they measured the pH at 6.2.
The TA was probably zero from tabs use.
It depends on the pH and chlorine levels.
Most likely, the customer uses trichlor tabs and the tab feeder sent corrosive liquid into the heat exchanger while the pump was off.
I had to add 34 drops of base reagent to get to a ph of 7.2 or so. What would you think the ph is based on that? To then estimate LSI?
I didn’t measure, but per orenda app, that seems to have only slight impact on LSI within a range of 10 to 100 all else being equal.CYA?
Was there a tab feeder?
What was the chlorine level?
What was the salinity?
The CYA matters, can you get that number?
Did you ask what was added to correct the low pH?
Did you ask what the TA was before the pH was corrected?
What was the chlorine level?
What was the salinity?
The CYA matters, can you get that number?
Did you ask what was added to correct the low pH?
Did you ask what the TA was before the pH was corrected?
Last edited:
The LSI does not really matter for copper corrosion, only for scaling.I didn’t measure, but per Orenda app, that seems to have only slight impact on LSI within a range of 10 to 100 all else being equal.
However, the CYA is super important to allow me to properly analyze the chemistry.
You should always test everything and keep a sample of water for reference for at least a week.
It was probably zero before the customer panicked and added a bunch of baking soda to try to correct the pH before you got there and voided the warranty.TA is 120 or so.
34 drops of base demand is equivalent to increasing the TA by 122.4 ppm.I had to add 34 drops of base reagent to get to a ph of 7.2 or so. What would you think the ph is based on that?
For a 10,000 gallon pool, that is 174 oz washing soda or 275 oz baking soda.
Most likely, the CYA is probably between 100 and 200, but we need to know for sure.



What probably happened is that a lot of baking soda was added and this created a lot of carbon dioxide.
The carbon dioxide and high CYA are causing a high level of Total Acidity, which is why the Base Demand was so high.
The Carbon Dioxide will offgass over the next few days and the pH should come up unless most of the total acidity is due to CYA over 150 ppm.
The current pH is probably between 5.5 and 6.8 but it is difficult to tell without a CYA reading.
You should keep a pH meter to read low pH like this.
HCO3- + H+ --> H2CO3 --> H2O + CO2.
CO2 + H2O <--> HCO3- + H+
HCO3- + H+ <--> CO2 + H2O

 aperainst.com
aperainst.com
 aperainst.com
aperainst.com

 aperainst.com
aperainst.com
The carbon dioxide and high CYA are causing a high level of Total Acidity, which is why the Base Demand was so high.
The Carbon Dioxide will offgass over the next few days and the pH should come up unless most of the total acidity is due to CYA over 150 ppm.
The current pH is probably between 5.5 and 6.8 but it is difficult to tell without a CYA reading.
You should keep a pH meter to read low pH like this.
HCO3- + H+ --> H2CO3 --> H2O + CO2.
CO2 + H2O <--> HCO3- + H+
HCO3- + H+ <--> CO2 + H2O

PH60 Premium Digital Pocket Waterproof pH Meter pH Pen Tester Kit with Replaceable Probe and Backlight-Apera Instruments
The Apera Instruments PH60 Premium Digital Pocket pH Meter / pH Pen Tester is ideal for all kinds of regular aqueous solution's testing, such as hydroponics, aquaculture, pools& spas, water treatment, cooling towers, etc. Can be replaced with ORP probe.
60 Series Portable Premium Pocket pH/EC/Salinity/TDS/ORP Meters and Testers
Apera Instruments 60 series portable premium pocket pH/EC/Salinity/TDS/ORP Meters and Testers are designed for regular multi-parameter measurements in soil, hydroponics, brewing, water testing, food and dairy products, and pools and spas.

PC60 Premium Multiparameter (pH/EC/TDS/Salinity/Temperature) Pocket Tester/Meter Kit-Apera Instruments
The Apera Instruments PC60 Multi-parameter Tester/Meter simply tests pH/EC/TDS/Salinity/Temperature for regular aqueous solutions such as hydroponics, aquaculture, pools& spas, tap/drinking water, water treatment, cooling towers, etc.
Likely to be inaccurate due to copper interference.CH is 1250, very high.

Copper Questions
The main takeaway is that at 0 TA, the pH is 4.5, but it drops fast after the TA is no longer available to buffer the effect of acid. You lose a full pH unit just by lowering the TA by 15 ppm. You hit a pH of 3 at -50 ppm.
I’ll go back in the morning to get CYA reading. And I just ordered the pH meter. Thanks!Likely to be inaccurate due to copper interference.
View attachment 627838
View attachment 627839
Copper Questions
The main takeaway is that at 0 TA, the pH is 4.5, but it drops fast after the TA is no longer available to buffer the effect of acid. You lose a full pH unit just by lowering the TA by 15 ppm. You hit a pH of 3 at -50 ppm.www.troublefreepool.com
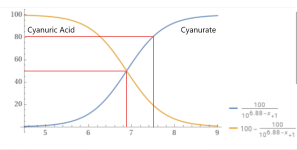
The pKa for CYA is about 6.88.
The pKa for carbonic acid is about 6.35.
Since the total acidity and total alkalinity are about equal, the pH is probably about 5.85 to 6.6.
So, basically, half of the CYA is acid and half is base (Cyanurate).
You have roughly equal amounts of Carbon dioxide and bicarbonate on a molar basis.
Total alkalinity prevents pH drop from added acid.
Total Acidity prevents pH rise from added base, which is what you measure with a base demand test.

 www.wolframalpha.com
www.wolframalpha.com

 www.wolframalpha.com
www.wolframalpha.com
y = 100/(10^(6.35 - x) + 1) and y = 100 - 100/(10^(6.35 - x) + 1), x = 4.5 to 8.3
graph y = 100/((10^(6.35-x)) + 1) and y = 100 - (100/((10^(6.35-x)) + 1)), x from 4.5 to 8.3
 www.wolframalpha.com
www.wolframalpha.com
The pKa for carbonic acid is about 6.35.
Since the total acidity and total alkalinity are about equal, the pH is probably about 5.85 to 6.6.
So, basically, half of the CYA is acid and half is base (Cyanurate).
You have roughly equal amounts of Carbon dioxide and bicarbonate on a molar basis.
Total alkalinity prevents pH drop from added acid.
Total Acidity prevents pH rise from added base, which is what you measure with a base demand test.


graph y = 100/((10^(6.88-x)) + 1) and y = 100 - (100/((10^(6.88-x)) + 1)), x from 4 to 9 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.

graph y = 100/((10^(6.35-x)) + 1) and y = 100 - (100/((10^(6.35-x)) + 1)), x from 5.3 to 8.3 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
y = 100/(10^(6.35 - x) + 1) and y = 100 - 100/(10^(6.35 - x) + 1), x = 4.5 to 8.3
graph y = 100/((10^(6.35-x)) + 1) and y = 100 - (100/((10^(6.35-x)) + 1)), x from 4.5 to 8.3
y = 100/(10^(6.35 - x) + 1) and y = 100 - 100/(10^(6.35 - x) + 1), x = 4.5 to 8.3 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
pH is probably between these red lines.
The pH should go up as the CO2 offgasses.
Like a soda or beer, the CO2 naturally goes from the water to the air.
At a pH of 5.85, the CO2 is about 76% on a molar basis compared to bicarbonate.
As the pH rises, the percentage of CO2 goes down and the percentage of HCO3 goes up.

 www.wolframalpha.com
www.wolframalpha.com
The pH should go up as the CO2 offgasses.
Like a soda or beer, the CO2 naturally goes from the water to the air.
At a pH of 5.85, the CO2 is about 76% on a molar basis compared to bicarbonate.
As the pH rises, the percentage of CO2 goes down and the percentage of HCO3 goes up.

100 - (100/((10^(6.35-5.85)) + 1)) - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
Using the Carbonic Acid and Bicarbonate Buffer, we get a starting pH of about 6.24.
pH1 = Start pH. (6.24174).
pH2 = 4.5
pH3 = 7.2
pH1 to pH2 = Total Alkalinity. Percentage bicarbonate at pH1 - Percentage bicarbonate at pH2 (0%).
pH1 to pH 3 = Total Acidity (Base Demand). Percentage CO2 at pH1 - Percentage CO2 at pH3 (12.4%)
Total Alkalinity = Total Acidity.
Percentage CO2 at pH1 = B
Percentage bicarbonate at pH1 = C.
B + C = 100
C = 100 -B
Total Alkalinity = C - 0.
Total Acidity (Base Demand) = B - 12.4
C = B - 12.4
100 - B = B - 12.4
100 + 12.4 = 2B
B = 56.2%
Start pH = 6.24174, which is 56.2% CO2 and 43.8% bicarbonate.
For the base demand (Total Acidity), you are going from CO2 at 56.2% to CO2 at 12.4%, which is a change of 43.8%. pH 7.2 - 6.24 = 0.96. Moving up by about 1 pH point.
For the Total Alkalinity, you are going from HCO3 at 43.8% to HCO3 at 0%, which is a change of 43.8%. ph 6.24- 4.5 = 1.74. Dropping by about 1.75 pH points.
pH1 = Start pH. (6.24174).
pH2 = 4.5
pH3 = 7.2
pH1 to pH2 = Total Alkalinity. Percentage bicarbonate at pH1 - Percentage bicarbonate at pH2 (0%).
pH1 to pH 3 = Total Acidity (Base Demand). Percentage CO2 at pH1 - Percentage CO2 at pH3 (12.4%)
Total Alkalinity = Total Acidity.
Percentage CO2 at pH1 = B
Percentage bicarbonate at pH1 = C.
B + C = 100
C = 100 -B
Total Alkalinity = C - 0.
Total Acidity (Base Demand) = B - 12.4
C = B - 12.4
100 - B = B - 12.4
100 + 12.4 = 2B
B = 56.2%
Start pH = 6.24174, which is 56.2% CO2 and 43.8% bicarbonate.
For the base demand (Total Acidity), you are going from CO2 at 56.2% to CO2 at 12.4%, which is a change of 43.8%. pH 7.2 - 6.24 = 0.96. Moving up by about 1 pH point.
For the Total Alkalinity, you are going from HCO3 at 43.8% to HCO3 at 0%, which is a change of 43.8%. ph 6.24- 4.5 = 1.74. Dropping by about 1.75 pH points.
Last edited:
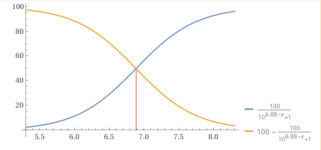
If we use the Cyanuric Acid and Cyanurate Buffer, we get a starting pH of 6.588.
We can estimate the pH at about 6.24 to 6.59 (6.415 average) depending on the amount of CYA in the water.
pH1 = Start pH. (6.588).
pH2 = 4.5
pH3 = 7.2
pH1 to pH2 = Total Alkalinity. Percentage Cyanurate at pH1 - Percentage Cyanurate at pH2 (0).
pH1 to pH 3 = Total Acidity (Base Demand). Percentage Cyanuric Acid at pH1 - Percentage Cyanuric Acid at pH3 (32.37%)
Total Alkalinity = Total Acidity.
Percentage Cyanuric Acid at pH1 = B
Percentage Cyanurate at pH1 = C.
B + C = 100
C = 100 -B
Total Alkalinity = C - 0.
Total Acidity (Base Demand) = B - 32.37
C = B - 32.37
100 - B = B - 32.37
100 + 32.37 = 2B
B = 66.185%
Start pH = 6.588, which is 66.185% Cyanuric Acid and 33.815% Cyanurate.
66.185 - 32.37 = 33.185.
7.2 - 6.59 = 0.61.
6.59 -4.5 = 2.09.
We can estimate the pH at about 6.24 to 6.59 (6.415 average) depending on the amount of CYA in the water.
pH1 = Start pH. (6.588).
pH2 = 4.5
pH3 = 7.2
pH1 to pH2 = Total Alkalinity. Percentage Cyanurate at pH1 - Percentage Cyanurate at pH2 (0).
pH1 to pH 3 = Total Acidity (Base Demand). Percentage Cyanuric Acid at pH1 - Percentage Cyanuric Acid at pH3 (32.37%)
Total Alkalinity = Total Acidity.
Percentage Cyanuric Acid at pH1 = B
Percentage Cyanurate at pH1 = C.
B + C = 100
C = 100 -B
Total Alkalinity = C - 0.
Total Acidity (Base Demand) = B - 32.37
C = B - 32.37
100 - B = B - 32.37
100 + 32.37 = 2B
B = 66.185%
Start pH = 6.588, which is 66.185% Cyanuric Acid and 33.815% Cyanurate.
66.185 - 32.37 = 33.185.
7.2 - 6.59 = 0.61.
6.59 -4.5 = 2.09.
Last edited:
For high CYA (Over 100) as the pH goes below about 6.9, you get a lot of total acidity (Base Demand) because most of the CYA is in the acid form.
You also get a lot of carbon dioxide.
This much CO2 does not last long, which indicates that it was created recently from someone adding a lot of baking soda to correct the 0 TA and the pH at below 4.5.
With a TA at 120, the pH will not be below 7.2 for long unless the CYA is over 300 ppm.

_____________________________________

You also get a lot of carbon dioxide.
That is a lot of base demand for a pH increase of 0.6 to 1.0 and it indicates that you have a lot of CO2 and probably a lot of CYA buffering the pH rise.I had to add 34 drops of base reagent to get to a ph of 7.2 or so.
This much CO2 does not last long, which indicates that it was created recently from someone adding a lot of baking soda to correct the 0 TA and the pH at below 4.5.
With a TA at 120, the pH will not be below 7.2 for long unless the CYA is over 300 ppm.

_____________________________________

Last edited:
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

