I guess this might get controversial, but I hope not. Before getting into it, a word of caution: If you decide there is merit to this and you therefore embark on a process to lower your TA levels,
please be very careful to watch your pH and TA levels so that you avoid the risk of equipment damage, surface damage, and swimmer irritation. Keep your pH above 7.0! Also, I am by no means an expert, I’m not a chemist, I just stumbled on to this recently after years of wasting time and money.
.... On to the reason for the post, my goal being constructive to maybe save some time and expense for many of us --------------
Have you ever raised your Total Alkalinity (TA) to a value near the center of the” recommended range” (eg 70 or 80) – only to find yourself adding muriatic acid (MA) over subsequent weeks to keep the PH from rising too high and too too fast? I was repeating that cycle of adding TA then MA for many years until I recently learned that it’s totally unnecessary. Aside from wasting time and money on TA & MA, there are other symptoms. Do you have an SWG cell that gets some buildup of calcium on a regular basis? Other surfaces with calcium buildup? You may be able to avoid that too, just by allowing your TA level to drop to the low end of the “recommended range” of 50-90. Other users have reported zero calcium buildup in their SWG cell and zero need for adding acid. That startled me! Have you installed and maintained an acid injection system to counter the pH rise? My recent discovery has me questioning whether that too is necessary.
There is at least one important caveat: If you’re using trichlor pucks to chlorinate, your water will be naturally more acidic, or if your water is otherwise naturally acidic for any reason, you might need that middle to upper end of the recommended TA range and you must test regularly to ensure your pH does not get dangerously low. Low pH can be dangerous to equipment, surfaces and swimmers. The
CDC says 7.0 to 7.8 here. TFP says
7.2 to 8.0 here.
More to the story: The purpose of having the correct Total Alkalinity level is well documented – in TFP and elsewhere - often stated this way: “to buffer against dramatic swings in PH”. While that’s true, I found a more complete definition. From
Orendatech and other sources, a more precise definition reads: “Total Alkalinity only buffers against a
reduction in pH”. As you dig around TFP and elsewhere on the internet, you’ll find plenty of tools and resources that imply the TA could or should be anywhere from 50-90, including the common quote stating “
50-90, sometimes higher” or the “ideal range” stated as 60-80. It is said that some pool stores and other resources recommend that TA be as high as 120. I guess that sells of lot of alkalinity increaser and muriatic acid! Included in my recent discovery is that those ranges are not the range for any pool. Rather, if you want to stop chasing your tail by constantly adding TA-then-MA,
you need to find the TA value that is appropriate for your pool. When you find the best TA value for your particular pool, you may find you can eliminate most of the TA-MA chemical add cycle. In the process, perhaps save on SWG cell replacement, acid injection equipment, etc.
There are two ways to summarize:
- One TFP user summarized it this way: “pH and TA management will be different for each pool, but I believe the simple approach is to manage pH and let TA settle where it wants - without totally ignoring it of course”. Credit to @AUSpool
- It’s worth reading more here – TA-Further Reading and notice the latter part of this paragraph I underlined: “Like pH, there is no “optimal” TA value - your optimal TA value is the value at which your pH is most stable (longest between acid additions). Typically, people find that a TA somewhere between 80-100ppm works well enough. However, if a pool experiences constant acid demand and the pH rises too quickly, TA can be safely lowered as far as 50 PPM to compensate for this. Despite what the “pool experts” will say, keeping your TA on the low end will not “rot out your equipment” or “destroy your plaster surfaces. “
Formerly while wallowing in my chemistry ignorance and not realizing that TA only buffers
against pH reduction, I read those recommended ranges as “push it to 90 or higher, then let it float back down gradually while adding acid (each week) to manage pH”. Then, a month or two or three later I would check and add TA again, restarting the mad cycle. Since I only tested pH and added acid weekly, among the effects was that my pH was rising to 7.8 or higher each week before I treated again with acid. It is said that the high pH probably caused the calcium buildup in the SWG cell. Contributing to my regular pH rise is also the aeration from my spa spillover waterfall, and it is said that the SWG cell provides some aeration effect as well. And it does not escape me, now, that the addition of acid is what brings down the TA in the first place. Ugh.
Is there any other reason for keeping TA higher, aside from buffering against pH reduction? I suppose not, a lesson learned. Well, we also manage CSI/LSI, but that is naturally managed through the rest of the chemistry.
If you’re interested in more reading, most of this came to light, ironically, for me and other TFP users in responses to this thread I started in the salt/SWG forum
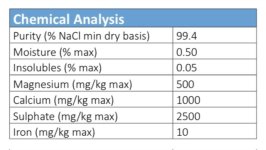
Iron stain from pool salt - all or most salts have iron.. Since my owners manual for the Hayward Aquarite SWG recommends checking & cleaning every 3 months, and since the controller alerts me to check the cell every 3 months, it never occurred to me that the calcium buildup could be avoided. In that thread you’ll find at least one other user experiencing what I experience, and other users whose SWG cells remain clean and clear of calcium – and they almost never need to add acid. Proper TA level seems to be the cure.
Hope this helped someone!