How Important is Free Chlorine in Pool Water?
Maintaining an appropriate FC level is the most important part of keeping your water clear and sanitary. Free chlorine is the primary sanitizer for pool water and one must maintain a proper residual concentration of chlorine in water at all times (it gets used up and must be replenished constantly).
When free chlorine levels drop too low or go to zero, algae and pathogens (bacteria, protozoa and viruses) are able to grow and replicate in your pool water. Pool water can have low FC levels and be hazardous to the health of swimmers even when the water is clear! This is why TFP will always say -
“Clear water is not a sign of clean water!”
What is the right Level of Free Chlorine in a Pool?
The level of FC you need to maintain depends on your CYA level and how much you use the pool. See the Chlorine/CYA Chart for guidelines on the appropriate FC level to maintain based on your CYA level.
What Are the Best Ways to Increase Free Chlorine in a Pool?
Recommended ways to raise FC include:
- household bleach (sodium hypochlorite, typically 8.25% in solution)
- liquid chlorine (higher concentrations of sodium hypochlorite)
- salt water chlorine generators (electrochemical production of chlorine gas from the chloride ion in salt).
Non-Recommended ways to raise FC:
- solid, stabilized chlorine compounds such as calcium hypochlorite (“cal-hypo”)
- lithium hypochlorite
- sodium dichloroisocyanurate (“dichlor”)
- trichloroisocyanuric acid (“trichlor”)
These solid compounds (tablets/pucks and bags of powder) will all add “extra” stuff that you do not want too much of in your water (eg, cal-hypo adds calcium, dichlor and trichlor add excess CYA, etc).
- For every 10 ppm Free Chlorine (FC) added by Trichlor, it also increases Cyanuric Acid (CYA) by 6 ppm.
- For every 10 ppm FC added by Dichlor, it also increases CYA by 9 ppm.
- For every 10 ppm FC added by Cal-Hypo, it also increases Calcium Hardness (CH) by 7 ppm.
Even at a very low chlorine usage of 1 ppm FC per day, using Trichlor as your only source of chlorine will increase CYA by over 100 ppm in just 6 months, unless there is significant dilution of the water. At 2 ppm FC per day usage, the CYA increases by over 100 ppm in just 3 months.
How Can Free Chlorine be Lowered in Pool Water?
In outdoor pools free chlorine will naturally decline 2-4 ppm a day due to the sun.
There are chlorine neutralizers most likely contain sodium thiosulfate.
- Add Sodium Thiosulfate, aka Chlorine Neutralizer for instant reduction.
- Add Ascorbic Acid, aka Vitamin C to remove chlorine.
- Add Hydrogen Peroxide, aka H2O2, to breakdown chlorine.
- Remove chlorine naturally with sunlight, aeration and agitation of the water.
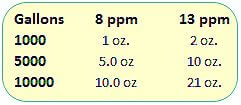
Sodium Thiosulfate, add 2 oz per 10,000 gallons of pool water to lower chlorine level by 1.0 ppm.Probably the cheapest and most reliable method to lower chlorine levels, in pools or spas.[1]
Ascorbic Acid – yes, granular Vit C! Add 32 oz per 10,000 gallons of pool water to lower chlorine level by about 9 ppm.
Hydrogen Peroxide – use 1 gallon of Aqua Silk Oxidizer per 10,000 gallons of pool water to lower chlorine level by 55 ppm. For spas, use 3% drug store peroxide, 2 ounces per 100 gallons of spa water, to lower chlorine (or bromine) level by about 10 ppm.
For Sodium Thiosulfate, add about a cup (8 oz) of the dry white crystals per 5,000 gallons of pool water, or 1 oz per 100 gallons – to lower chlorine by 10 ppm. Check your pool water pH and adjust to within label instructions, and add directly to the pool or spa.
Sodium thiosulfate dosage In pools and spas, you want to be careful not to overshoot the mark, or overdose the pool with Sodium Thio, or you may have difficulty adding new chlorine, temporarily at least. Sodium Thiosulfate will dissipate from most pools within a few days. Follow the label instructions carefully when using a chlorine remover, for recommended water balance and dosages.
Another good resource is this sodium thiosulfate calculator on Koiphen.com – enter your pool size in gallons (or liters) and the current chlorine level in ppm, and it tells you how many ounces or grams of Sodium Thiosulfate to add to reduce chlorine to zero.
What is Free Chlorine in a Pool?
Free chlorine, as measured by a TFP recommended test kit, is the sum of two forms of chlorine in your pool water - active chlorine (the chlorine compounds that directly oxidize bather waste and kill pathogens) and reserve chlorine (the chlorine that is bound to the cyanuric acid stabilizer, or CYA).
What is Active Chlorine in a Pool?
Active chlorine compounds come in two forms - hypochlorous acid (HOCl) and the hypochlorite ion (OCl-). Both forms of active chlorine will oxidize and disinfect but hypochlorous acid is the more potent form of active chlorine while the hypochlorite ion is more susceptible to loss from UV radiation (it absorbs UV and gets turned into the inert form of chlorine, the chloride ion or Cl-).
What is Reserve Chlorine in a Pool?
Reserve chlorine compounds come in many different chemical forms but they can all be classified as chlorinated cyanurates (sometimes denoted as HCy-Cl). Basically, the chlorine atom has attached itself to the cyanuric acid molecule and it is “held” in reserve and protected from UV loss. As the active chlorine compounds get used up by disinfection and oxidation, the chlorinated cyanurates release the chlorine atoms to form more active chlorine.
What is the Difference Between Active and Reserve Chlorine?
A good analogy for the role of active and reserve chlorine is this - think of the chlorine in your pool as an army. The solders on the front lines fighting the enemy are your active chlorine species. The reserve chlorine species are your soldiers waiting behind the front lines ready to replace an active soldier that dies in combat.
Which Types of Chlorine are Being Tested?
Free chlorine is an aggregate value of three distinct forms of chlorine -
FC = [HCy-Cl] + [HOCl] + [OCl-] Free chlorine = "chlorine bound to CYA" + Hypochlorous acid + Hypochlorite anion[2]
When CYA is present in pool water above 30ppm or so, almost 95% of the chlorine in the water is bound to the CYA molecule where it is not reacting with anything. The test of the chlorine splits up into hypochlorous acid (HOCl) and hypochlorite anion (OCl-). Those two chlorine species are often called "active chlorine" as they are the chemical agents responsible for oxidation and disinfection. HOCl is what kills algae & bacteria and inactivates viruses, and it is also responsible for the oxidation of organic compounds. Hypochlorite anion is a weak oxidizer and disinfectant but sit strongly reacts with UV light and is reduced to oxygen gas and chloride ion (Cl-). As the HOCl and OCl- get used up, more chlorine is released from the CYA to maintain an equilibrium balance. So chlorine bound to CYA is often referred to as "reserve chlorine". So in this way, CYA acts as a buffer for chlorine's reactive species.
Because of the chemistry involved, the DPD dye reacts to all three forms of "active chlorine" and so you are measuring an aggregate value. There is no way to measure the three species separately outside of a very sophisticated chemistry lab, so the simpler value of FC is what is used.
Why Do Others Warn About Chlorine Levels That TFP Recommends?
The problem is, the "harshness of chlorine" depends on the HOCl level mostly and so basing recommendations on FC without considering CYA is illogical and why the industry has such a poor track record at helping people maintain clean and clear swimming pools.
Active chlorine levels are different than Free Chlorine levels. As long as your FC/CYA ratio is between 5% and 40%, the water is safe to swim in from a chlorine perspective. Considering the FC/CYA ratio is important because we do not have a direct way of measuring the Active chlorine.
Why don't they make chlorine tabs without the added CYA
Chlorine is a gas. It must be trapped by some material for a residential pool owner to use it. The most stable and economically viable are:
- Cyanuric Acid - TriChlor,
- Calcium - Calcium Hypochlorite, or
- Water - sodium hypochlorite (aka bleach or Liquid Chlorine).
There are cal-hypo tablets that do not contain CYA. You need a special feeder for them. They will add calcium to your water whoch can cause other unwanted problems as the pool CH rises. [[Calcium Hardness}Calcium]] is the same as CYA, it does not go away without draining the pool water.
That is why a Salt Water Chlorine Generator is so easy and appropriate for a residential pool owner. You create your chlorine on site. Only thing you use is electricity. The device that creates the chlorine will last years if properly sized and water chemistry is properly maintained.
How Does pH and CYA Effect Free Chlorine?
So what determines how much, or the proportion of active chlorine to reserve chlorine in your pool?
Basically, it depends on the pH of your water (more about that later) and the concentration of cyanuric acid (CYA). Typically speaking, most of the free chlorine (more than 90% of it at normal pool pH) is held in reserve by the cyanuric acid and the rest of the free chlorine (hypochlorous acid and hypochlorite ion) break up into proportions dictated by pH (at a pH of 7.5, the amounts of HOCl to OCl- are 1:1 but, in total, only a small fraction of the overall FC).
The most important point to remember though is this - the amount of active chlorine ion your pool water is determined by the concentration of CYA. You need enough CYA in your water to create a proper reserve of chlorine, BUT, too much CYA can reduce the amount of active chlorine in your water to levels too low to fight pathogens and oxidize bather waste. This is called “overstabilization” of pool water and, mistakenly, is referred to as “chlorine lock” by the pool industry due to them not acknowledging the importance of the CYA/FC ratio.
How Can Algae Grow in Water With Chlorine?
Algae replication rates in warm water with insufficient sanitizer can be high enough such that there is a colony doubling every 2 to 4 hours (depends on species, light requirements, etc). So in a 24 hour period with light available for more than 12 hours per day (summer time), algae grows very fast. The kill rate of any sanitizer is concentration dependent.[3]
There's not a lot of hard data on the CT kill rate for algae with chlorine, at least no easily available data, but once your active chlorine levels (that would be the hypochlorous acid part of the FC) fall below 100 part per billion (0.1ppm), the kill rates are well below the colony doubling times. So basically, algae can freely grow and thrive when there is (insufficient) sanitizer around.
Despite what you might think, your water is not one, homogenous mixture. While your water sample might say there's 0.5ppm or 1ppm FC, there's a very good chance that water right at the surface of the pool has no chlorine in it whatsoever (UV light destroys chlorine quickly) and the water nearest the walls probably has much lower FC levels.
Does Chlorine Consumption Change with Water Temperature?
Warmer water losing more FC due to sunlight than colder water is not true.[4]
Chlorine loss from the UV in sunlight does not depend on temperature because it only depends on the number of photons per area entering into the pool and on the concentration of chlorine. The photons of light are traveling much faster (at the speed of light) than the molecules containing chlorine so the temperature which relates to the speed of those chlorine molecules is irrelevant. From the point of view of the photons, the molecules of chlorine are essentially standing still so the cross-section of those molecules which is the area with which the photon has a quantum probability of reacting with the molecule is independent of the temperature and only related to the concentration of such molecules in the water.
Chlorine consumption that is related to temperature is for chemical reactions with chlorine such as oxidizing pool covers, bather waste, pollen, leaves, algae, etc. And yes, algae grows faster in warmer water but such consumption won't matter if there is sufficient FC/CYA since algae will get killed before it can reproduce so the rate will be based solely on the rate of blown-in algae spores which is usually not measurable (pollen, on the other hand, can be voluminous as can pods and other material dropped from trees).
A pool with a solar cover that is opaque to UV would lower the loss of chlorine from sunlight that is not temperature dependent but would increase the loss of chlorine from oxidizing the cover which is temperature dependent.
There can be a temperature dependence on the subsequent chemical reactions that occur after the photochemical reaction occurs.[5] But for the breakdown of chlorine by UV, it's pretty much all over with such breakdown since the probability of having the OH• and Cl• reform compared to other reactions that lead forming chloride and oxygen gas is low and not temperature dependent. At most, the intermediate concentrations of some intermediate species such as hydrogen peroxide may be higher at lower temperatures, but by that time it's too late and the chlorine is already on it's way to becoming chloride.
There can be a small dependence on temperature in terms of the rate of relaxation from excited states so there is a small but negligible temperature dependence on having the HO-Cl vibrational state be excited and not break apart as often because of low temperature, but it takes a very low temperature before that effect would be seen in practice.[6]
Can FC Targets be Lowered for Pools Open in Cold Weather?
Though the growth rate of algae slows down as water gets colder, the reaction of chlorine killing algae also slows down. So lowering the FC level too much could be risky.
The main advantage of the cooler water, especially if the sun isn't on the pool due to clouds or a cover, is the lower daily FC usage. I'd keep the FC at whatever level you normally need to do to prevent algae.
Though it's possible that at some cold water temperature the algae growth plummets, why take the chance that it doesn't?[7]
- ↑ https://www.troublefreepool.com/threads/yep-a-new-guy-probably-not-new-problems.198891/post-1756025
- ↑ https://www.troublefreepool.com/threads/chlorine-levels-is-it-ever-unsafe-if-it-is-in-range-of-your-cya.211252/post-1851556
- ↑ https://www.troublefreepool.com/threads/understanding-algae.190879/post-1681395
- ↑ https://www.troublefreepool.com/threads/temperature-and-chlorine-consumption.98645/post-851170
- ↑ http://www.tandfonline.com/doi/pdf/10.1080/00022470.1961.10468041
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/15969616
- ↑ https://www.troublefreepool.com/threads/algae-and-cool-water.26599/post-224531