You know, I just found this thread after asking a
very similar question about Cl- build-up. Everything makes sense but I'm hung up on something:
To be pedantic, I'm not sure this is correct. I'm pretty sure
the water does that. Only the Cl- participates in the reaction in the salt sell; the Na+ is needed to make the water conductive. You don't actually need the sodium to make hypochlorous acid but the electrolysis would be impossible without it. In theory, you could use other chloride salts to get the same effect. I hope someone will correct me if I'm wrong about this.
Ok here we go, continuing the thought from above. When you say "salt" here (and when we test salt levels in the pool) most people probably think "table salt" like
@JJ_Tex said, but I assume we're using the
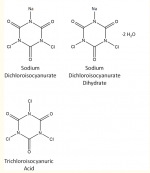
chemistry definition and if my understanding is correct, the sodium doesn't really matter. If you use bleach or chlorinating liquid, that's NaClO (sodium hypochlorite) and it will provide enough Na+ to make this work like you describe. But if you use dichlor or trichlor pucks, those don't contain any sodium. Is there some other positively-charged ion the Cl- will bond with in this case?
Because if not, if you run your pool on things like tabs and cal-hypo for a long time and never introduce any sodium, then you decide to convert to salt... is the excessive amount of existing Cl- going to cause a problem?
I know we don't like those products here (for good reason) but I come across so many people who swear by them. And these days with the national shortage and everyone converting to salt, I'm thinking this could be a very plausible scenario.