- Jul 3, 2015
- 175
- Pool Size
- 38500
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Jandy Aquapure 1400
Remember the basics of what we teach. Chlorine is consumed by either organics in your pool or UV from the sun. Since you eliminate the sun during the OCLT, the only possible explanation for your 1.5 ppm loss is organics (which you don't want) in your pool.. is there something I’m missing???!!!
Can you read heads or tails?
You must stay the course until the FC kills enough of the organics (and you clean and brush and backwash to get them removed) that you will pass the OCLT. You are close.
Always good to remove any metal from the pool.*can I leave the quarters in the pool or do they need to be fished out?
Will do. Thanks.Always good to remove any metal from the pool.
Uh. Not an easy task without diving for them. ?Will do. Thanks.
Oh NO, @mariane! But... awwwwww ?.Maybe ducks are swimming in your pool.
That happened to me, why the water was clear and failed OCLT. The ducks were having an early morning swim!
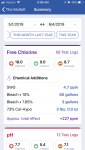
 to the MAX!!!
to the MAX!!!
If it is ammonia, the pH and TA will drop as chlorine is added.
So, that can help confirm that you have ammonia.
Get your system operational, get the pH to below 8 and begin SLAM.
Monitor TA as you go to see if it drops during the SLAM.
I suspect that you probably have ammonia and hydrogen sulfide.




