So I understand the need to increase your free chlorine levels as your cya increases. But my question is what happens to that chlorine that is bound to the cya? Is it essentially useless? Does it unbind to kill bacteria? Because we increase our free chlorine because most of it is bound to CYA does this mean the free chlorine isn’t protected by CYA? Also is the chlorine not bound to CYA subject to PH to determine its strength? Sorry lots of questions
Help me understand the PH CYA and Chlorine relationship
- Thread starter CapnCrunch
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- May 3, 2014
- 62,687
- Pool Size
- 6000
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-40
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
Chlorinated cyanurate and unbound chlorine are in an equilibrium system. With given values for FC, CYA and pH, there are well defined amounts of each chlorine species.
Without CYA, there are two chlorine species showing up in the FC test. Hypochlorous acid (HOCl) and hypochlorite ion (OCl-). At low pH, mainly HOCl, at pH 7.5 both at about 50%, at high pH mainly OCl-. HOCl is the powerful sanitizer and oxidizer that we are interested in, which is why without CYA one should target a pH below 7.6
When adding CYA, more than 95% of the chlorine is now attached to CYA, where it is protected from UV light, but has no sanitizing and oxidizing powers anymore. It's only the chlorine that is not attached to CYA which is killing bacteria.
But unfortunately the chlorine that's bound to CYA still shows up as FC in the test.
That's why you need to adjust FC to the CYA level, so that the remaining 5% of "real free" or "active" chlorine provide sufficient amounts of HOCl. The HOCl concentration is proportional to the FC/CYA ratio. In other words, FC 12 / CYA 30 and FC 24 / CYA 60 provide same amounts of HOCl, which is the same amount that FC 0.64 with CYA 0 provides.
The chlorinated cyanurates provide basically a chlorine reservoir. As soon as HOCl and OCl- get used up by UV or killing "stuff", more gets released from the reservoir until a new equilibrium between all the species is established (which happens pretty much instantaneously).
The reservoir also has an effect on the pH dependency of the HOCl concentration, with rising pH more HOCl gets released from the reservoir, compensating to a large degree the change of HOCl into OCl- with rising pH. The relation between just HOCl and OCl- remains the same, but the HOCl concentration is also determined by the equilibrium with the chlorinated cyanurates, and this altogether plays out in a way the the HOCl contraction is now less pH-dependant.
Without CYA, the HOCl concentration decreases by 50% from pH 7.5 to 8.0. With CYA this decrease is only 15%.
CYA is basically what makes an outdoor residential pool manageable without fancy equipment.
Without CYA, you should actually maintain FC between about 0.2 and 0.6 ppm to have safe, but pleasant water. They do that for example in public pools in Europe. But you need basically real-time testing and dosing to maintain these levels. Just one kid peeing in a small residential pool would be enough to crash FC down to zero.
With CYA you can maintain a "macroscopic" FC level (like 5 or 10, pretty much anything up to SLAM-FC for a given CYA), that feels like a "microscopic" level. If that same kid now "kills" say 0.5 ppm FC, then some more "active" chlorine gets released from the reservoir, and in the end your FC just went down from say 5 to 4.5 (a 10% change, rather than 100% if 0.5 was all that you had), and the HOCl also only went down by the same 10%.
CYA is pretty awesome when used properly.
Without CYA, there are two chlorine species showing up in the FC test. Hypochlorous acid (HOCl) and hypochlorite ion (OCl-). At low pH, mainly HOCl, at pH 7.5 both at about 50%, at high pH mainly OCl-. HOCl is the powerful sanitizer and oxidizer that we are interested in, which is why without CYA one should target a pH below 7.6
When adding CYA, more than 95% of the chlorine is now attached to CYA, where it is protected from UV light, but has no sanitizing and oxidizing powers anymore. It's only the chlorine that is not attached to CYA which is killing bacteria.
But unfortunately the chlorine that's bound to CYA still shows up as FC in the test.
That's why you need to adjust FC to the CYA level, so that the remaining 5% of "real free" or "active" chlorine provide sufficient amounts of HOCl. The HOCl concentration is proportional to the FC/CYA ratio. In other words, FC 12 / CYA 30 and FC 24 / CYA 60 provide same amounts of HOCl, which is the same amount that FC 0.64 with CYA 0 provides.
The chlorinated cyanurates provide basically a chlorine reservoir. As soon as HOCl and OCl- get used up by UV or killing "stuff", more gets released from the reservoir until a new equilibrium between all the species is established (which happens pretty much instantaneously).
The reservoir also has an effect on the pH dependency of the HOCl concentration, with rising pH more HOCl gets released from the reservoir, compensating to a large degree the change of HOCl into OCl- with rising pH. The relation between just HOCl and OCl- remains the same, but the HOCl concentration is also determined by the equilibrium with the chlorinated cyanurates, and this altogether plays out in a way the the HOCl contraction is now less pH-dependant.
Without CYA, the HOCl concentration decreases by 50% from pH 7.5 to 8.0. With CYA this decrease is only 15%.
CYA is basically what makes an outdoor residential pool manageable without fancy equipment.
Without CYA, you should actually maintain FC between about 0.2 and 0.6 ppm to have safe, but pleasant water. They do that for example in public pools in Europe. But you need basically real-time testing and dosing to maintain these levels. Just one kid peeing in a small residential pool would be enough to crash FC down to zero.
With CYA you can maintain a "macroscopic" FC level (like 5 or 10, pretty much anything up to SLAM-FC for a given CYA), that feels like a "microscopic" level. If that same kid now "kills" say 0.5 ppm FC, then some more "active" chlorine gets released from the reservoir, and in the end your FC just went down from say 5 to 4.5 (a 10% change, rather than 100% if 0.5 was all that you had), and the HOCl also only went down by the same 10%.
CYA is pretty awesome when used properly.
Last edited:
Gotta Pool
Well-known member
I wouldn't ever suggest that you not read the articles cited, but a straightforward answer is:
97% or more of the chlorine is bound to the CYA and the remaining portion is in equilibrium. As that small portion is consumed in pool sanitation it is replenished by the chlorine bound to the CYA until the chlorine is depleted. The chlorine dissipated by the sun and the chlorine actively involved in sanitation is consumed.
pH affects the amount of chlorine as HOCl (the best killing agent) and OCl-. IOW, the closer the pH is to 7 the the better the killing power of the chlorine. Of course, that low pH brings a host of other problems. If you have a green pool, keeping the pH at about 7.2 can help, but probably isn't the best swiming water.
CYA and TA (and if applicable, Borates) help to buffer the pH, too.
97% or more of the chlorine is bound to the CYA and the remaining portion is in equilibrium. As that small portion is consumed in pool sanitation it is replenished by the chlorine bound to the CYA until the chlorine is depleted. The chlorine dissipated by the sun and the chlorine actively involved in sanitation is consumed.
pH affects the amount of chlorine as HOCl (the best killing agent) and OCl-. IOW, the closer the pH is to 7 the the better the killing power of the chlorine. Of course, that low pH brings a host of other problems. If you have a green pool, keeping the pH at about 7.2 can help, but probably isn't the best swiming water.
CYA and TA (and if applicable, Borates) help to buffer the pH, too.
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
pH affects the amount of chlorine as HOCl (the best killing agent) and OCl-. IOW, the closer the pH is to 7 the the better the killing power of the chlorine. Of course, that low pH brings a host of other problems. If you have a green pool, keeping the pH at about 7.2 can help, but probably isn't the best swiming water.
The pH effect is quite small in the presence of CYA as I have explained in my answer above.
The reservoir also has an effect on the pH dependency of the HOCl concentration, with rising pH more HOCl gets released from the reservoir, compensating to a large degree the change of HOCl into OCl- with rising pH. The relation between just HOCl and OCl- remains the same, but the HOCl concentration is also determined by the equilibrium with the chlorinated cyanurates, and this altogether plays out in a way the the HOCl contraction is now less pH-dependant.
Without CYA, the HOCl concentration decreases by 50% from pH 7.5 to 8.0. With CYA this decrease is only 15%.
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
The pH effect is quite small in the presence of CYA
Maybe a few graphs to make that a bit clearer.
Let's start with this one:
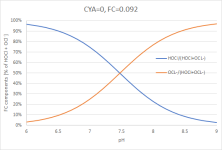
(Graph 1)
This is what everyone has in mind when saying that pH affects chlorine effectiveness. And this plot - with concentrations of HOCl and OCl⁻ plotted as percentages relative to the sum of the HOCl- and OCL⁻-concentrations (which in case of CYA=0 is just FC) - remains valid whenever you have chlorine dissolved in water.
Now we plot that (still no CYA involved) not as a percentage, but in concentrations:
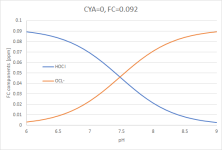
(Graph 2)
Pretty much the same plot as before with ppm (or mg Cl₂ / L) instead of %. I have chosen FC=0.092 because it yields [HOCl] = 0.05ppm (I am using the notation [...] for concentrations which is usually used for mol/L, but it makes the writing a bit easier), which is a typical TFP target range [HOCl], at a pH of 7.4 (which is the centre of the "classical" range between 7.2 and 7.6).
Now I am adding 30ppm of CYA to the equation and bump up FC to an appropriate target level, which changes Graph 2 to:
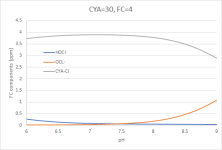
(Graph 3)
Most of the FC is now attached to CYA, called "CYA-Cl" in the graph. Zooming in on the bottom two curves:
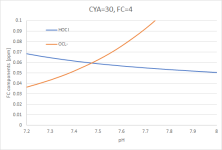
(Graph 4)
You can see that the pH-dependency of [HOCl] is now much flatter. At pH 8.0, [HOCl] is 0.05ppm - the same as with FC=0.092/CYA=0 at pH=7.4 (which was the reason why I have chosen the odd number of 0.092 for the example).
But when plotting [HOCl] and [OCl⁻] not as absolute concentrations or relative to FC, but relative to just the sum of [HOCl] and [OCl⁻], you get exactly the same picture as above (Graph 1) again:
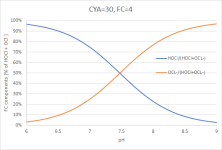
(Graph 5)
The equilibrium between [HOCl] and [OCl⁻] hasn't changed, but it's not the only relevant equilibrium anymore.
To summarise, a final plot of [HOCl] for CYA=0/FC=0.092, CYA=30/FC=4 and CYA=80/FC=10.3 all in one graph. Both scenarios with CYA yield the same [HOCl] at pH=8.0 as CYA=0/FC=0.092 at pH=7.4:
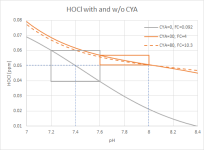
(Graph 6)
You can see how much flatter the pH-dependency of [HOCl] is with CYA. It allows pH-maintenance between 7.6 and 8.0, while staying within a much narrower [HOCl] band compared to maintaining pH between 7.2 and 7.6 without CYA.
Even at pH=8.4 you are still well above the grey bottom limit (but I wouldn't recommend to target such high pH for metal staining and calcium scaling reasons, and you have relatively high concentrations of OCl⁻ - see Graph 4 - at this stage that is more susceptible to UV-decay than HOCl - one of the reasons why we advise to lower pH before a SLAM, not because of chlorine efficiency, but because of higher losses to UV).
You can see that CYA allows pool operation in a pH-area that is a lot less affected by pH-rise due to CO₂ outgassing.
You can also see how little HOCl you actually need to keep a pool sanitized and algae free, and that the barn gate "classical" range of FC between 1 and 10ppm regardless of CYA is absolute insanity. It allows under- and over-chlorination to disgusting degrees.
So when someone makes you a compliment that you have such a clean pool with obviously hardly any chlorine, then they are actually absolutely right: You have very little of the chlorine species that matters. The bulk is kept in storage.
Last edited:
Gotta Pool
Well-known member
You didn't refute anything I said. The verbose, graphic, and subtle nuances of this scientific inquiry were posited by ChemGeek (Richard Falk) and Ben Powell fifteen years ago and relegated to "The Deep End." The full-blown explanations should probably stay there. I was trying to answer valid and simple questions with equally simple and valid answers.
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
Your straightforward answer is appreciated, but your focus on pH was giving a wrong message.
Keeping pH at 7.2 is not what's really important to clear a green pool. Adding chlorine is. An initial pH-reduction before adding large amounts of chlorine is required to mitigate high pH during a SLAM, but keeping pH at 7.2 is not a focus. The pH test is skewed at high FC and advising to maintain 7.2 during a SLAM can lead to overcorrecting pH.
I admittedly went a bit too deep, and you are right that nothing of this is new. I just wanted to reiterate that even though the correlation between HOCl and OCl- remains unchanged, the overall pH-dependency of HOCl is fairly insignificant with CYA.
Keeping pH at 7.2 is not what's really important to clear a green pool. Adding chlorine is. An initial pH-reduction before adding large amounts of chlorine is required to mitigate high pH during a SLAM, but keeping pH at 7.2 is not a focus. The pH test is skewed at high FC and advising to maintain 7.2 during a SLAM can lead to overcorrecting pH.
I admittedly went a bit too deep, and you are right that nothing of this is new. I just wanted to reiterate that even though the correlation between HOCl and OCl- remains unchanged, the overall pH-dependency of HOCl is fairly insignificant with CYA.
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

