Hi, you obviously know more about chemistry than I do so you've caused me to hit "pause". I guess I need to get deeper into this. Everything you have said is consistent with what I am learning except what you have said about the DPD test. Assuming the DPD test does what you say, and it includes bound as well as unbound it is indicating "potential" FC and not actual unbound FC actively sanitizing. Is that how you see itt?Both DPD and FAS/DPD tests measure the active FC, HOCl, the chlorinate ion, OCl-, and the chlorine bound to CyA as FC in ppm. More accurately the chlorine bound to CyA are chlorinated cyanurates. The reactions between HOCl and OCl-, and the reactions between HOCl and CyA are all equilibrium reactions that are in constant flux. As a little bit of HOCl is used roughly the same amount of Is “unbound” from the CyA maintaining equilibrium. Reaction rates or change in reactants and products to maintain equilibrium are very quick. Equilibrium is maintained but the ratio slowly changes as HOCl is used, the ratio is corrected when we add more FC.
Other then water volume and chlorine strength another big factor that will cause variations between calculated and measured FC is lingering bacteria, algae and general other waste that can use a significant potion of the added FC.
CYA not considered in Pool Math?
- Thread starter DougJack
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Interesting - I've tried the same thing and it hasn't updated for me. I'm actually now using an SWG but that isn't modifying my curiosity.I played more with poolmath and it seems to want all 3 values added each time. (Current FC / CYA / target FC). If you just change the CYA without adding random #s for the other 2, the recommedation doesn't update. Sometimes entering new values for current FC and CYA will update the recommendations, but usually not without target FC.
I forwarded the bug up the chain with a PM and email.
I'd use the chart for now until you know what the levels should be from memory.
Hi Steve, Of course not being a chemist, I am relying a lot on AE as I try to avoid going too far down the rabbit hole. I set up a basic question to get a more specific answer on what the FAS-DPD test was measuring with this: Imagine you are a research chemist. You have a hot tub and want to measure the FC available for the sanitizing. You have a Taylor FAS-DPD test kit. When you measure FC you know you are measuring the unbonded HOCL and OCL-, but are you measuring also the disassociated FC from the CYA or also measuring the HOCL (isocyanurate) that is the bonded FC? -Both DPD and FAS/DPD tests measure the active FC, HOCl, the chlorinate ion, OCl-, and the chlorine bound to CyA as FC in ppm. More accurately the chlorine bound to CyA are chlorinated cyanurates. The reactions between HOCl and OCl-, and the reactions between HOCl and CyA are all equilibrium reactions that are in constant flux. As a little bit of HOCl is used roughly the same amount of Is “unbound” from the CyA maintaining equilibrium. Reaction rates or change in reactants and products to maintain equilibrium are very quick. Equilibrium is maintained but the ratio slowly changes as HOCl is used, the ratio is corrected when we add more FC.
Other then water volume and chlorine strength another big factor that will cause variations between calculated and measured FC is lingering bacteria, algae and general other waste that can use a significant potion of the added FC.
Gemini came back with this which is just their conclusion:
Conclusion
- The FAS-DPD test primarily measures free HOCl/OCl- and a portion of the rapidly dissociating chlorine from CYA. It gives a good indication of the available sanitizing chlorine.
- It does not measure the total amount of chlorine bonded to the CYA.
- To obtain a more complete picture, we'd need to consider the equilibrium dynamics and potentially use complementary measurement techniques.
So It seems to be a bit of a hybrid and represents the local and quick sanitizing potential.
- Jul 21, 2013
- 65,068
- Pool Size
- 35000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
So when real people tell you the AI is wrong who do you choose to believe?Hi Steve, Of course not being a chemist, I am relying a lot on AE as I try to avoid going too far down the rabbit hole. I set up a basic question to get a more specific answer on what the FAS-DPD test was measuring with this: Imagine you are a research chemist. You have a hot tub and want to measure the FC available for the sanitizing. You have a Taylor FAS-DPD test kit. When you measure FC you know you are measuring the unbonded HOCL and OCL-, but are you measuring also the disassociated FC from the CYA or also measuring the HOCL (isocyanurate) that is the bonded FC? -
Gemini came back with this which is just their conclusion:
Conclusion
As a research chemist, we understand that "measuring" is often an approximation of reality, and our goal is to understand the limitations and refine our methods.
- The FAS-DPD test primarily measures free HOCl/OCl- and a portion of the rapidly dissociating chlorine from CYA. It gives a good indication of the available sanitizing chlorine.
- It does not measure the total amount of chlorine bonded to the CYA.
- To obtain a more complete picture, we'd need to consider the equilibrium dynamics and potentially use complementary measurement techniques.
So It seems to be a bit of a hybrid and represents the local and quick sanitizing potential.
Please argue with your robot and not us.
Hi Steve, Just another comment intended to reduce variables. I have an interesting cover that raises straight up while makiing a light/UV blocking o-ring seal over the hot tub. Once a day, or more when I'm home, I will open the hot tub, turn on the jets for 5 minutes and then take a sample 1 foot down from the same location and measure at 90 F or lower - usually around 85 F. The drop in FAS-DPD is around .5 ppm/day, so little residual organic load even if the tub wasn't used 24 hours before. I can get it to drop about twice as fast if I open the lid, probably a combination of outgassing and reflected light.Both DPD and FAS/DPD tests measure the active FC, HOCl, the chlorinate ion, OCl-, and the chlorine bound to CyA as FC in ppm. More accurately the chlorine bound to CyA are chlorinated cyanurates. The reactions between HOCl and OCl-, and the reactions between HOCl and CyA are all equilibrium reactions that are in constant flux. As a little bit of HOCl is used roughly the same amount of Is “unbound” from the CyA maintaining equilibrium. Reaction rates or change in reactants and products to maintain equilibrium are very quick. Equilibrium is maintained but the ratio slowly changes as HOCl is used, the ratio is corrected when we add more FC.
Other then water volume and chlorine strength another big factor that will cause variations between calculated and measured FC is lingering bacteria, algae and general other waste that can use a significant potion of the added FC.
I'm not arguing, I'm pursuing clarity. Please don't take this as an argument in any emotional sense.So when real people tell you the AI is wrong who do you choose to believe?
Please argue with your robot and not us.
Imagine you are a research chemist.Imagine you are a research chemist.
Does that make you a research chemist?
Just because you tell AI to imagine they are something, it does not automatically make them that thing.

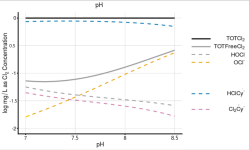
log0.00007748M = -4.11 for CYA = 10 ppm.
log(1.41 x 10^-5) = -4.85 = 1 ppm for Total Cl2.
The HOCl and OCl- bind to the reagent and the chlorine bonded to the CYA does not combine with the reagent.
However, as the HOCl and OCl- get used up, the chlorine bonded to the CYA gets released.
In an equilibrium, there are always chlorine atoms bonding and unbonding to the CYA and the net result is a specific percentage of HOCl and OCl- are free to bond to the indicator reagent and get neutralized by the titrating reagent.
The net result is that all chlorine is measured in the test.
Below is a calculator you can use.
Last edited:
Simulation for 7ppmFC/70ppmCyA

________________________________________________________________________________________________________________
FC = 1 and CYA = 10 ppm.
________________________________________________________________________________________________________________
FC = 10 and CYA = 100.
Total Free Cl2 at pH = 7.5 = 0.08 ppm = 0.8% of FC.

________________________________________________________________________________________________________________

________________________________________________________________________________________________________________
FC = 1 and CYA = 10 ppm.

________________________________________________________________________________________________________________
FC = 10 and CYA = 100.
Total Free Cl2 at pH = 7.5 = 0.08 ppm = 0.8% of FC.

________________________________________________________________________________________________________________
A buffered DPD indicator powder is added to a water sample and reacts with chlorine (HOCl and OCl-) to produce the pink color characteristic of the standard DPD test.
Ferrous ammonium sulfate (FAS) is then added drop by drop until the pink color completely and permanently disappears, signaling the endpoint of the reaction.
Ferrous ammonium sulfate is considered a "reducing agent" because it readily donates electrons in chemical reactions, meaning it can reduce other substances by causing them to gain electrons; this property is primarily due to the presence of iron in its +2 oxidation state (ferrous ion) which can easily be oxidized to the +3 state (ferric ion) by accepting electrons.
The chlorine is reduced to chloride and this causes chlorine to be released from the CYA molecule and the released chlorine is then available to react with the reagents.
This goes until all chlorine is consumed.
The release is fast enough so that all of the chlorine is measured in the test.
Ferrous ammonium sulfate (FAS) is then added drop by drop until the pink color completely and permanently disappears, signaling the endpoint of the reaction.
Ferrous ammonium sulfate is considered a "reducing agent" because it readily donates electrons in chemical reactions, meaning it can reduce other substances by causing them to gain electrons; this property is primarily due to the presence of iron in its +2 oxidation state (ferrous ion) which can easily be oxidized to the +3 state (ferric ion) by accepting electrons.
The chlorine is reduced to chloride and this causes chlorine to be released from the CYA molecule and the released chlorine is then available to react with the reagents.
This goes until all chlorine is consumed.
The release is fast enough so that all of the chlorine is measured in the test.
- Jul 21, 2013
- 65,068
- Pool Size
- 35000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
No emotion involved by me on you opinion shopping between real people and robots.I'm not arguing, I'm pursuing clarity. Please don't take this as an argument in any emotional sense.
When you run out of your own thoughts you turn to robots and bring it here.
Explain what YOU do not understand about what has been explained you. Not what you imagine happens.
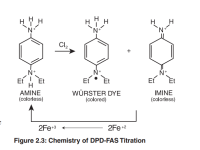
The chemical basis for the DPD chlorine reaction is depicted in Figure 2.1.
The DPD amine is oxidized by chlorine to two oxidation products.
At a near neutral pH, the primary oxidation product is a semi-quinoid cationic compound known as a Würster dye.
This relatively stable free radical species accounts for the magenta color in the DPD colorimetric test.
DPD can be further oxidized to a relatively unstable, colorless imine compound.
When DPD reacts with small amounts of chlorine at a near neutral pH, the Würster dye is the principal oxidation product.
At higher oxidant levels, the formation of the unstable colorless imine is favored — resulting in apparent “fading”of the colored solution.


2b. DPD Titration Method.
The DPD titration method is based on the same chemistry as the DPD colorimetric method – in that DPD is oxidized by chlorine (or iodine in the case of chloramines) to the magenta-color species.
The red color then is titrated with a ferrous reducing agent to the colorless end point.
The reaction chemistry is depicted in Figure 2.3.
The DPD amine is oxidized by chlorine to two oxidation products.
At a near neutral pH, the primary oxidation product is a semi-quinoid cationic compound known as a Würster dye.
This relatively stable free radical species accounts for the magenta color in the DPD colorimetric test.
DPD can be further oxidized to a relatively unstable, colorless imine compound.
When DPD reacts with small amounts of chlorine at a near neutral pH, the Würster dye is the principal oxidation product.
At higher oxidant levels, the formation of the unstable colorless imine is favored — resulting in apparent “fading”of the colored solution.



2b. DPD Titration Method.
The DPD titration method is based on the same chemistry as the DPD colorimetric method – in that DPD is oxidized by chlorine (or iodine in the case of chloramines) to the magenta-color species.
The red color then is titrated with a ferrous reducing agent to the colorless end point.
The reaction chemistry is depicted in Figure 2.3.
Attachments
How DPD Measurement Works
DPD stands for N,N-diethyl-p-phenylenediamine, and is the name of a colourless compound that will turn a bright pink colour when in the presence of chlorine as well as other oxidising agents.
When DPD reacts with chlorine it is oxidised to form a Würster dye (a compound with a distinct magenta colour), as shown in the reaction scheme below.
The solution formed has a distinct magenta colour, and the amount of Würster dye formed is proportional to the amount of free chlorine present.
Secondary oxidation of the Würster dye causes a colourless imine product to form.
At chlorine levels above the range of the DPD test chosen, the colourless imine will be formed and so the pink colour will start to fade and can even disappear.
This is known as the bleaching effect.
In these cases, a dilution is required to bring the sample back within the range of the test.

 www.palintest.com
www.palintest.com
DPD stands for N,N-diethyl-p-phenylenediamine, and is the name of a colourless compound that will turn a bright pink colour when in the presence of chlorine as well as other oxidising agents.
When DPD reacts with chlorine it is oxidised to form a Würster dye (a compound with a distinct magenta colour), as shown in the reaction scheme below.
The solution formed has a distinct magenta colour, and the amount of Würster dye formed is proportional to the amount of free chlorine present.
Secondary oxidation of the Würster dye causes a colourless imine product to form.
At chlorine levels above the range of the DPD test chosen, the colourless imine will be formed and so the pink colour will start to fade and can even disappear.
This is known as the bleaching effect.
In these cases, a dilution is required to bring the sample back within the range of the test.

Measuring Chlorine using DPD - Palintest
DPD continues to be the standard method of chlorine determination throughout the world. Here we review how DPD measurement works and what the options are when measuring chlorine using DPD. History of DPD Measurement The DPD colorimetric method is the result of extensive work carried out by Dr...
The amount of Würster dye produced from the DPD reagent is directly proportional to the amount of chlorine in the sample.
The colored dye is responsible for the absorbance at 520nm.
The colored dye is responsible for the absorbance at 520nm.
I decided to act a bit on your question about who do you trust - and I continuously have to challenge AI, so I understand the question and respect it. Directionally I find AI really useful. At any rate, I decided to search on google scholar for a paper that would summarize similar, but not exact work in this area. They concluded as you said, that all of the chlorinated isocyanurates were included in the DPD measurement. The purpose of the paper was multisided, but they started with a validated with high confidenceI'm not arguing, I'm pursuing clarity. Please don't take this as an argument in any emotional sense.
So, basically, the free chlorine (HOCl and OCl-) oxidizes the DPD to form a Würster dye with a magenta color.
As the free chlorine is used up oxidizing the DPD, more HOCl and OCl- are created until all of the chlorine is released from the CYA.
The Würster dye is then reduced by the FAS reagent (Titration) until the Würster dye is all reduced giving the amount of FC based on the number of drops of FAS reagent.
As the free chlorine is used up oxidizing the DPD, more HOCl and OCl- are created until all of the chlorine is released from the CYA.
The Würster dye is then reduced by the FAS reagent (Titration) until the Würster dye is all reduced giving the amount of FC based on the number of drops of FAS reagent.
I decided to act a bit on your question about who do you trust - and I continuously have to challenge AI, so I understand the question and respect it. Directionally I find AI really useful. At any rate, I decided to search on google scholar for a paper that would summarize similar, but not exact work in this area. They concluded as you said, that all of the chlorinated isocyanurates were included in the DPD measurement. The purpose of the paper was multisided, but they started with a validated with high confidence a simple model and then used that to predict actual FC in the water compared to measured. One of their conclusions was that DPD measured FC was biased by basically the total amount of bonded 'CYA. This implication (to me) is that at a nominal CYA level of 30, the actual FC is roughly only 5% of what is measured. Here is the paper link: Chlorinated Cyanurates in Drinking Water: Measurement Bias, Stability, and Disinfectant Byproduct Formation - PMC
- Jul 21, 2013
- 65,068
- Pool Size
- 35000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
I think you will discover TFP Scholar is more reliable and less work than most other sources., I decided to search on google scholar for a paper ..,
Hi - that is consistent with the paper I just read. They said that a DPD test measures all bonded HOCL and this is a measurement bias. I take this to mean that FC that is immediately available for sanitizing is only the part that isn't bound, or about 5% of the measured DPD if you have unsaturated CYA.View attachment 629503
log0.00007748M = -4.11 for CYA = 10 ppm.
log(1.41 x 10^-5) = -4.85 = 1 ppm for Total Cl2.
The HOCl and OCl- bind to the reagent and the chlorine bonded to the CYA does not combine with the reagent.
However, as the HOCl and OCl- get used up, the chlorine bonded to the CYA gets released.
In an equilibrium, there are always chlorine atoms bonding and unbonding to the CYA and the net result is a specific percentage of HOCl and OCl- are free to bond to the indicator reagent and get neutralized by the titrating reagent.
The net result is that all chlorine is measured in the test.
Below is a calculator you can use.
Attachments
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

