Sulfamic Acid is a strong acid (pKa =1.0) and completely dissociates in an aqueous solution.
H2NSO3H + H2O <==> H3O+ + H2NSO3-
So, the hydrogen attached to the oxygen should come off right away as the sulfamic is added to water.
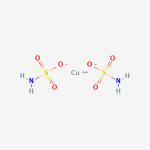
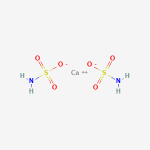
Copper or calcium can connect to the oxygen where the hydrogen was.
Since they are +2, they connect to two sulfamic acid molecules.
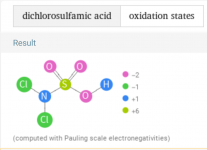
I think that the chlorine will mostly connect to the nitrogen as shown below.
The below shows the oxidation states, but I think that the chlorine will be in the +1 state and the Nitrogen will be in the -3 state until the chlorine oxidizes the nitrogen, at which time it can go to nitrogen gas at a 0 state or to nitrite or to nitrate.
UV probably helps break down the bonds by energizing the electrons and giving them a better chance of going to the chlorine atoms pulling on them.
H2NSO3H + H2O <==> H3O+ + H2NSO3-
So, the hydrogen attached to the oxygen should come off right away as the sulfamic is added to water.
Copper or calcium can connect to the oxygen where the hydrogen was.
Since they are +2, they connect to two sulfamic acid molecules.
I think that the chlorine will mostly connect to the nitrogen as shown below.
The below shows the oxidation states, but I think that the chlorine will be in the +1 state and the Nitrogen will be in the -3 state until the chlorine oxidizes the nitrogen, at which time it can go to nitrogen gas at a 0 state or to nitrite or to nitrate.
UV probably helps break down the bonds by energizing the electrons and giving them a better chance of going to the chlorine atoms pulling on them.








