- Jan 16, 2017
- 33
- Pool Size
- 14140
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool Edge-25
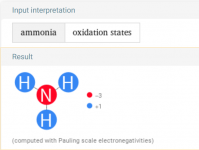
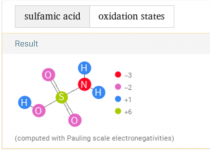
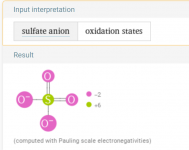
Borax it is thenWashing soda is also know as sodium carbonate or soda ash.
Borax is sodium tetraborate.
So something is chewing thru my chlorine and dropping my pH
This am
FC 3.0. CC 5.0 pH 7.2 TA 70
Added 2 gallons of bleach
This pm
FC 4.5 CC 3.0 pH 7.0 TA 60
Is the problem all the bleach I’m adding to keep the TC at 20 for the SLAM?