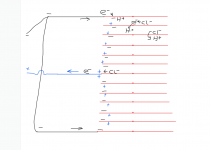
Note that the efficiency will not be 100%, so you have to account for that, but I don't know exactly what the efficiency should be for this process with this water chemistry.
There will be some oxygen produced, but I don't know how much compared to the production of chlorine.
The oxygen is still an oxidizer, so it might do some work that chlorine would do anyway.
For a 13 blade cell, below is the maximum theoretical chlorine production (Assuming that I am doing the calculations correctly).
The Hayward T-15 is a 13 blade cell and it has amperage in the 6 to 7 range for typical operation.
That puts the theoretical production at 2.5 to 2.91 lbs per day but Hayward says that the cell is rated for 1.47 lbs per day.
So, the question is, why does Hayward say 1.47 lbs per day?
Is the process 100% efficient?
Probably not.
1.47 lbs per day is about 1/2 of the possible output at 7 amps.
Does that suggest that the process is only 50% efficient?
Maybe Hayward has made an error in their calculations or testing or maybe a typo?
Maybe Hayward is being extra conservative to avoid liability?
Amps..........lbs per day
5.0..............2.08
5.2..............2.17
5.4..............2.25
5.6..............2.33
5.8..............2.42
6.0..............2.50
6.2..............2.58
6.4..............2.67
6.6..............2.75
6.8..............2.83
7.0..............2.91
7.2..............3.00
7.4..............3.08
7.6..............3.17
7.8..............3.25
T-15 has 13 Titanium Plates, 150 x 63mm. Produces 1.47 lbs/day (Per Hayward rating).
T-9 has 13 Titanium Plates, 101 x 63mm. Produces 0.98 lbs/day.
T-5 has 7 Titanium plate, 150 x 63mm. Produces 0.735 lbs/day.
T-3 has 7 Titanium Plates, 101 x 63mm. Produces 0.53 lbs/day.
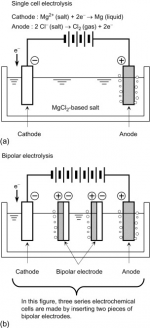
For the 7 plate cells, you have one set of six cells in series.
The below reference shows 30 grams per hour at 5 amps, which is 720 grams per day or 1.59 lbs. vs the theoretical amount of 2.08 lbs. per day, which implies an efficiency of about 76% assuming they are using a 13 plate cell.
For an 11 plate cell, 5 amps should produce about 1.73 lbs per day.
If they are using an 11 plate cell, then it is about 1.59/1.73 = 92% efficient.
The IC40 uses 11 plates, which means that you multiply the amps at the power supply by 5 vs 6.
So, 6 amps at the power supply is 30 amps total for the IC40.
6 amps at an Intellichlor power supply makes as much chlorine as 5 amps at an Aquarite T-15 power supply.




 While amps are an important factor, the amount of chlorine produced can also vary by manufacture, type cell, and other variables. Did you have a specific make/model SWG in mind? We have had several discussion on the forum this season about chlorine "advertised" versus the actual amount we've seen produced. In many cases, the amount of chlorine produced seems to fall short of what a manufacture is advertising, but if you take the advice of purchasing an SWG rated at 2X the size of your pool it generally does a good job meetings your FC demands.
While amps are an important factor, the amount of chlorine produced can also vary by manufacture, type cell, and other variables. Did you have a specific make/model SWG in mind? We have had several discussion on the forum this season about chlorine "advertised" versus the actual amount we've seen produced. In many cases, the amount of chlorine produced seems to fall short of what a manufacture is advertising, but if you take the advice of purchasing an SWG rated at 2X the size of your pool it generally does a good job meetings your FC demands.