- Sep 4, 2016
- 38
- Pool Size
- 44000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Zodiac LM3-40
Not sure if this post should be here or in the advanced topics section. My apologies if this is the wrong place. Mods please move.
Over the last year or so my SWG is making more and more flakes of scale and I cannot work out why. My CSI has always between -0.2 and -0.3 until recently where I've been lowering my pH more to try and remove HCO3 and have a lower CSI to prevent the precipitation in the SWG. Also my CYA is falling. I thought it was supposed to stay in solution and the problem was removing it if you're not following the TFP and adding a chlorine product with CYA. My problem is needing to replace it regularly. My salt and Ca are stable so it's not due to water loss and dilution. CYA was 80 on December 1 and is now 20, a loss of 60ppm over approximately 90 days. This makes me think CYA must be a component in the precipitation. My SWG is still making chlorine although is needing about a 20% higher setting or runtime. There's a link below showing what my robot picked up with about 5 days between runs.
I'm at a loss. I don't want to spend a heap of money on a new cell (or whole SWG unit) to find it's a chemistry problem.

 photos.app.goo.gl
photos.app.goo.gl
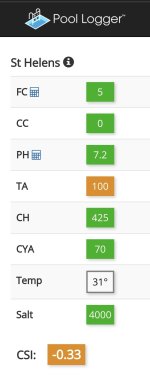
Over the last year or so my SWG is making more and more flakes of scale and I cannot work out why. My CSI has always between -0.2 and -0.3 until recently where I've been lowering my pH more to try and remove HCO3 and have a lower CSI to prevent the precipitation in the SWG. Also my CYA is falling. I thought it was supposed to stay in solution and the problem was removing it if you're not following the TFP and adding a chlorine product with CYA. My problem is needing to replace it regularly. My salt and Ca are stable so it's not due to water loss and dilution. CYA was 80 on December 1 and is now 20, a loss of 60ppm over approximately 90 days. This makes me think CYA must be a component in the precipitation. My SWG is still making chlorine although is needing about a 20% higher setting or runtime. There's a link below showing what my robot picked up with about 5 days between runs.
I'm at a loss. I don't want to spend a heap of money on a new cell (or whole SWG unit) to find it's a chemistry problem.
2 new items by Peter Bainbridge
 photos.app.goo.gl
photos.app.goo.gl

Last edited:


