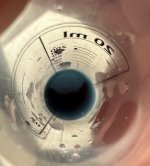
Only thing is that it's in spanish and my spanish is non-existant, even though I think I can guess what 'cloro' means

Just turn on the automatic translation that chrome offers in the browser menu in the top right corner (not the web page menu), it's surprisingly good these days:
My pool is indoors so the sun wont break down the chlorine. As I understood it I shouldn't need CYA in my pool if It's indoors? Please correct me if I'm wrong

TFP recommends to add a little CYA (about 20-30ppm) to indoor pools as a chlorine strength moderator:

www.troublefreepool.com
CYA changes the chemical equilibriums. Without CYA there is only a pH dependent equilibrium between hypochlorous acid (HOCl) and hypochlorite ion (OCl
-). At low ph, it's mostly HOCl, at high pH mostly OCl
-. HOCl is the one that has excellent sanitising and oxidising properties, that's why the pool industry recommends to maintain pH below 7.5.
With CYA, most of the chlorine (Let's say 95-97%) forms a chemical bond with CYA as chlorinated cyanurates. In this bond, chlorine is protected from UV light, but doesn't have any disinfecting properties. Unfortunately, it still shows up as FC in the tests, and that's where the confusion starts.
Regulators in Europe (I know that Germany is doing it that way, not sure about the Netherlands) react to this by just banning CYA outright in public pools. But at the same time they acknowledge that in this case FC should be kept below 0.6pmm (and above 0.3ppm).
In the US, the effect of CYA is largely being ignored. At low levels, it's UV protection is appreciated, but the effect on the chlorine efficiency is not understood and ignored, until at CYA 100 a mysterious "chlorine lock" occurs.
TFP follows the way of actually tackling this at the root. After Ben Powell found experimental evidence that FC needs to be adjusted to the CYA level, Richard Falk (aka chem geek on the forum here) looked up the relevant scientific publications (it all goes back to an O'Brien paper from 1972), and provided an explanation for Ben's observations. Details are all in chem geek's sticky threads in the Deep End forum.
In a nut shell: To maintain same HOCl concentrations, the FC/CYA ratio must be kept constant. That's it, the results are summarised here in the recommended
FC/CYA Levels.
The level of FC 0.6ppmm that is considered the upper limit in Germany is roughly equivalent to TFP's SLAM-level (for example FC 20 at CYA 50 or FC 16 at CYA 40 - same FC/CYA ratio). Everything below SLAM is considered safe for swimming. And everything below the minimum level is will result very quickly in an algae outbreak and is borderline unsanitary. Therefore maintain FC within the recommended target ranges.
The big advantage that maintaining FC in the target range (lets say FC 6-8ppm for CYA 50ppm) offers vs maintaining FC in the appropriate range between 0.3 and 0.6ppm without CYA is, that the latter is bloody impossible in a residential setup without fancy, expensive testing and dosing equipment. Let's say you have a couple of kids swimming that are too excited to go the the bathroom, and you will have lost all your 0.6ppm very quickly - urine eats up chlorine in no-time. Or a very sunny day with an outdoor pool. When losing 0.6ppm from a level of 6ppm, you are still within your target range, and miles ahead of your minimum level. The chlorinated cyanurates are basically providing a chlorine reservoir. Chlorine in the reservoir is protected from UV and basically not reacting with anything. In other words, it doesn't feel "harsh".
And this is the effect, that an indoor pool will also benefit from. You don't need it for UV protection, but a small level will allow you to keep FC within an easy to maintain target range, or in an even bigger range up to SLAM-FC - remember that SLAM is equivalent to about 0.6ppm without CYA which is considered the upper swimmable level under German regulations for public pools.
CYA also adds the benefit that it flattens the pH-dependency of the HOCl concentration. Without CYA, from pH 7.5 to pH 8.0, the HOCl concentration decreases by about 50% by turning into OCl
-. With CYA, it only decreases by about 15% (as HOCl turns into OCl
- with rising pH, more chlorine gets released from the reservoir, compensating for most of the loss. Therefore, with CYA,, at the appropriate
FC/CYA Levels, pH can be maintained anywhere that has a "7" in front of the decimal point. Much easier, with the added benefit that pH will stop rising so fast when allowed to settle a bit higher in combination with lower TA-levels.
The "high" levels of TA 100-120 recommended by the industry are only required when using Trichlor for chlorination as that keeps pulling down pH and TA. Using liquid chlorine or a Salt Water Chlorine Generator is pH- and TA-neutral, and lower TA-levels are possible, and even beneficial as it slows down the constant pH-rise due to CO
2 outgassing (high TA means more CO
2 dissolved in the water that wants to escape like in an opened bottle of soda water).
I hope I haven't overwhelmed you with my short story of nearly everything you need to know about pool care. Have a read through the Pool School articles, browse through the forums and fire away with your questions.
 so, could anyone please take a look at the results and tell me if:
so, could anyone please take a look at the results and tell me if: