This part is interesting.At pH 7, you have for example an H+ concentration of 10-7 mol/L and an OH- concentration of also 10-7 mol/L.
At pH 6, you have an H+ concentration of 10-6 mol/L and an OH- concentration of 10-8 mol/L.
At pH 8, you have an H+ concentration of 10-8 mol/L and an OH- concentration of 10-6 mol/L.
Purpose of lower TA
- Thread starter xDom
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Sep 23, 2015
- 2,012
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V25
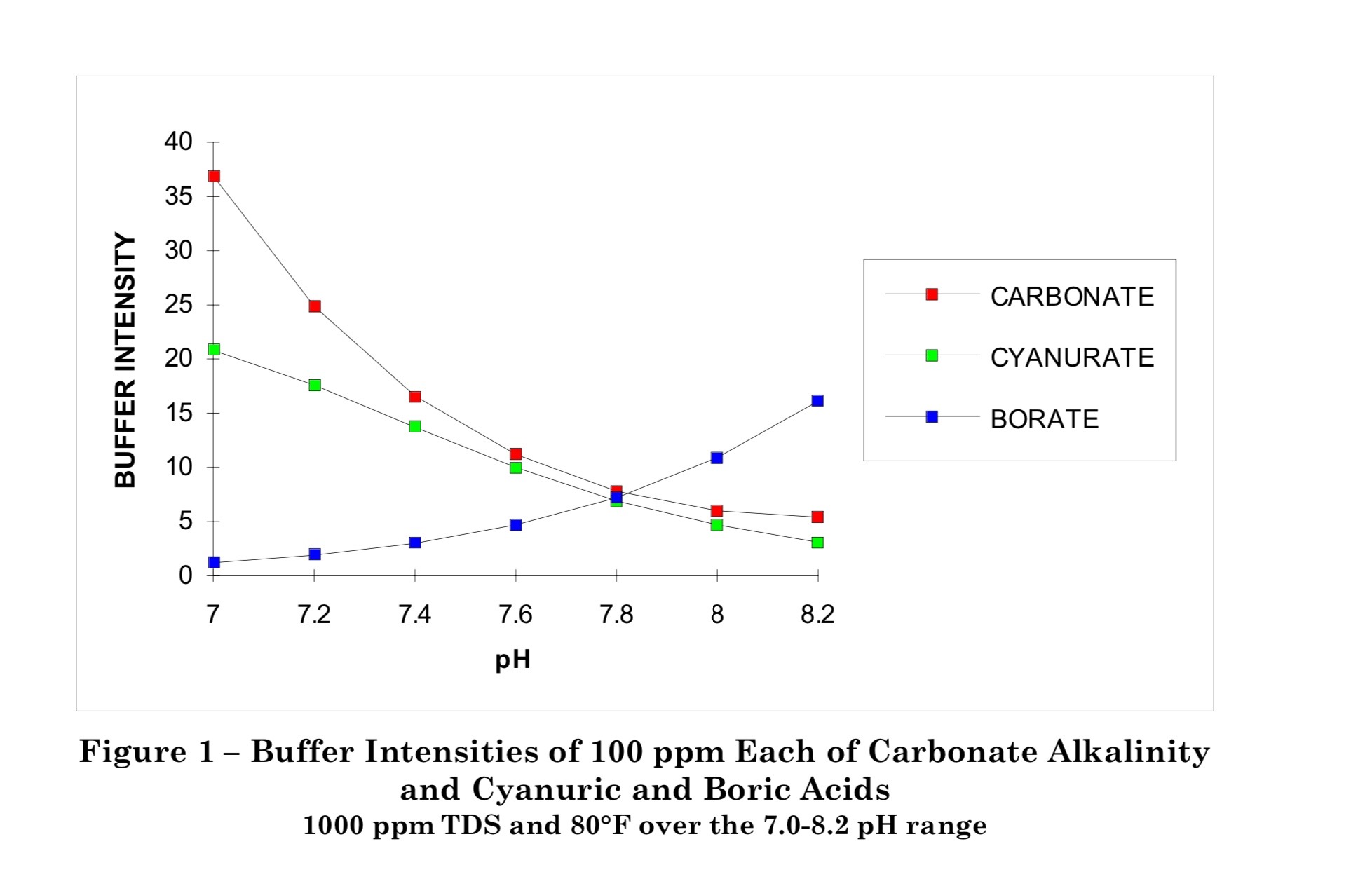
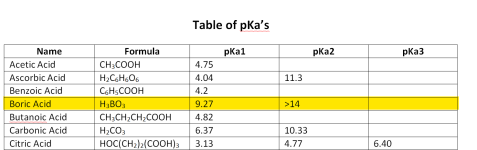
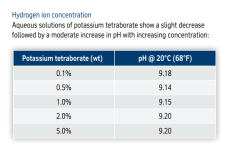
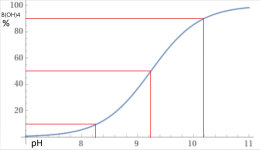
Carbonic acid has 2 pKas for the hydrogen ions; one at 6.3 and one at 10.3.
Below is the graph of how much bicarbonate converts to carbonate based on the pH.
Because 8.3 is the midpoint, you get the same amount of carbonic acid and carbonate when you add bicarbonate.
You get about 1% carbonate ions and about 1% carbonic acid or carbon dioxide at a pH of 8.3.
This is why baking soda always pushes the pH towards 8.3.
Given that the carbonate buffer system is pushing pool pH to 8.3, to equilibrium with carbonic acid and carbonate. With borates and a pKa of 9.2 for the boric acid / borate buffer system does this mean that the borate buffer system if used is pushing pool water to a pH of 9.2?
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
I think the difference is that the bicarbonate ion can both, accept and release, a proton, so it depends on the starting pH which effect dominates and which way pH goes when adding bicarb.
When adding borates via boric acid, then pH can't go up, as boric acid can't accept further protons.
When adding borates via boric acid, then pH can't go up, as boric acid can't accept further protons.
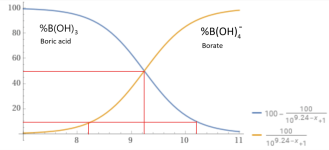
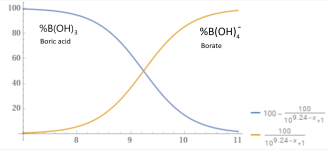
Acidity (pKa) 9.24 (9.14) (first proton), 12.4 (second), 13.3 (complete)
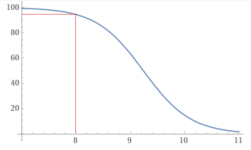
At a pH of 8.0 about 95% is in the form boric acid.
100B(OH)3 + 5H2O --> 95B(OH)3 + 5B(OH)4- + 5H+
 www.wolframalpha.com
www.wolframalpha.com
At a pH of 8.0 about 95% is in the form boric acid.
100B(OH)3 + 5H2O --> 95B(OH)3 + 5B(OH)4- + 5H+

y = 100-(100/(1+10^(9.24– x))), x from 7 to 11 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
Boric acid is like adding carbon dioxide or carbonic acid; it will lower the pH but it will not change the TA.
Borate is like baking soda and it will tend to push the pH towards the center of the first and second pKa.
Acidity (pKa) 9.24 (9.14) (first proton), 12.4 (second), 13.3 (complete)
If we use 9.14 and 12.4, then the center is about 10.8.
So, adding borate will tend to push the pH towards 10.8.
However, different sources show different numbers for the pKa2 of boric acid.
BH3O3 --> BH2O3- + H+ (pka1 9.14 or 9.24).
B(OH)3 + H2O --> B(OH)4- + H+ (pka1 9.14 or 9.24).
BH2O3- --> BHO32- + H+ (pka2 12.4).
BHO32- --> BO33- + H+(pka3 13.3).
BO33- + H2O --> BO2- +2OH-
_________________________________________________________________________________
_________________________________________________________________________________

Borate is like baking soda and it will tend to push the pH towards the center of the first and second pKa.
Acidity (pKa) 9.24 (9.14) (first proton), 12.4 (second), 13.3 (complete)
If we use 9.14 and 12.4, then the center is about 10.8.
So, adding borate will tend to push the pH towards 10.8.
However, different sources show different numbers for the pKa2 of boric acid.
BH3O3 --> BH2O3- + H+ (pka1 9.14 or 9.24).
B(OH)3 + H2O --> B(OH)4- + H+ (pka1 9.14 or 9.24).
BH2O3- --> BHO32- + H+ (pka2 12.4).
BHO32- --> BO33- + H+(pka3 13.3).
BO33- + H2O --> BO2- +2OH-

_________________________________________________________________________________

_________________________________________________________________________________



Last edited:
Using the following pKas, I would expect the addition of borate to push the pH towards 10.99.
(9.24 + 12.74)/2 = 10.99.
If the pH is over 10.99, the borate should lose a hydrogen ion, which will push the pH down.
If the pH is under 10.99, the borate should gain a hydrogen ion, which will push the pH up.
BH2O3- --> BHO32- + H+ (pH over 10.99).
BH2O3- + H+ --> BH3O3 (pH under 10.99).
pKa1 - 9.24,
pKa2 - 12.74,
pKa3 - 13.80
____________________________________________________________________________________________________________
____________________________________________________________________________________________________________
____________________________________________________________________________________________________________

____________________________________________________________________________________________________________
____________________________________________________________________________________________________________
____________________________________________________________________________________________________________
(9.24 + 12.74)/2 = 10.99.
If the pH is over 10.99, the borate should lose a hydrogen ion, which will push the pH down.
If the pH is under 10.99, the borate should gain a hydrogen ion, which will push the pH up.
BH2O3- --> BHO32- + H+ (pH over 10.99).
BH2O3- + H+ --> BH3O3 (pH under 10.99).
pKa1 - 9.24,
pKa2 - 12.74,
pKa3 - 13.80
____________________________________________________________________________________________________________

____________________________________________________________________________________________________________

____________________________________________________________________________________________________________

____________________________________________________________________________________________________________

____________________________________________________________________________________________________________

____________________________________________________________________________________________________________
Last edited:
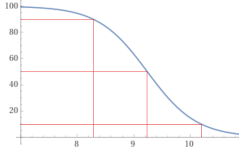
At a pH of 10.194, about 10% of added Borax (Sodium Tetraborate decahydrate) will become boric acid, which will lower the pH.
10(Na2B4O7 • 10H2O) --> 36B(OH)4- + 4B(OH)3 + 20Na+ + 14H2O + 16H+
However, if you use trisodium orthoborate, the pH will rise.
10Na3BO3 + 30H2O --> 30Na+ + 9B(OH)4- + B(OH)3 + 21OH-
_____________________________________________________________________
At a pH of 8.28, about 90% of added Borax will become boric acid, which will raise the pH.
10(Na2B4O7 • 10H2O) --> 4B(OH)4- + 36B(OH)3 + 20Na+ + 30H2O + 16OH-
_____________________________________________________________________
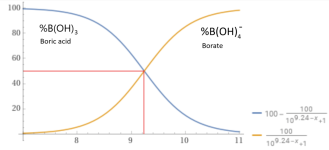
At a pH of 9.24, about 50% of added Borax will become boric acid and 50% will become borate, which will be pH neutral.
Na2B4O7 • 10H2O --> 2B(OH)4- + 2B(OH)3 + 2Na+ + 3H2O
_____________________________________________________________________
 www.wolframalpha.com
www.wolframalpha.com
10(Na2B4O7 • 10H2O) --> 36B(OH)4- + 4B(OH)3 + 20Na+ + 14H2O + 16H+
However, if you use trisodium orthoborate, the pH will rise.
10Na3BO3 + 30H2O --> 30Na+ + 9B(OH)4- + B(OH)3 + 21OH-
_____________________________________________________________________
At a pH of 8.28, about 90% of added Borax will become boric acid, which will raise the pH.
10(Na2B4O7 • 10H2O) --> 4B(OH)4- + 36B(OH)3 + 20Na+ + 30H2O + 16OH-
_____________________________________________________________________
At a pH of 9.24, about 50% of added Borax will become boric acid and 50% will become borate, which will be pH neutral.
Na2B4O7 • 10H2O --> 2B(OH)4- + 2B(OH)3 + 2Na+ + 3H2O
_____________________________________________________________________

y = 100-(100/(1+10^(9.24– x))), x from 7to 11 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
Last edited:
The bicarbonate creates carbon dioxide, which offgasses until the pH gets to about 8.3, this is why the pH rises automatically to about 8.3.With borates and a pKa of 9.2 for the boric acid / borate buffer system does this mean that the borate buffer system if used is pushing pool water to a pH of 9.2?
Baking soda can become carbonic acid or carbonate and this happens equally at a pH of about 8.3.
This is why baking soda pushes the pH towards 8.3 when it is added.
For added Borax, the pH gets pushed to the pKa as shown in the previous post.
However, the borate buffer does not produce a gas like carbonate does, so there is no automatic push towards the pKa except when adding Borax.
If you add boric acid, it will lower the pH but not the TA.
y = (100/(1+10^(9.24– x))), x from 7 to 11 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
It is a myth that SWGs cause pH rise.
Anode 4Cl- -> 2Cl2.
Cathode 4H2O -> 2H2 + 4OH-.
2Cl2 +2H2O -> 3H+ + HOCl + OCl-.
HOCl + OCl- + uv light -> O2 + H+ + 2Cl-.
Following the process, we can see that there are 4H+ and 4OH- created, which nets out to pH neutral.
The chlorine gas generated is very acidic and creates 3 hydrogen ions for every 4 hydroxide ions created.
As the hypochlorous acid is broken down by UV, 1 more hydrogen ion is created for a net neutral result.
Assuming that chlorine gain and loss are equal, there's no pH rise.
However, there is hydroxide generated in the cell, which can cause scaling due to the production of calcium carbonate.
Ca2+ + HCO3- + OH- --> CaCO3 +H2O
If you have B(OH)3 (Boric acid) in the water, it buffers the pH change by taking some of the hydroxides as the pH rises.
Borates to contribute to the TA (Total Alkalinity), but their most important role is in providing Total Acidity, which is the resistance to pH rise from added base (Like hydroxide), whereas total alkalinity is the resistance to pH drop from added acid.
Ca2+ + HCO3- + OH- + B(OH)3 --> Ca2+ + HCO3- + B(OH)4-
Reducing the TA means that there is less Carbon dioxide, bicarbonate and carbonate in the water.
Carbon dioxide causes pH rise as it offgasses.
Bicarbonate and carbonate can create scale in the cell as it produces chlorine.
The general pool industry way overestimates the need to have TA as a buffer to pH change.
 www.wolframalpha.com
www.wolframalpha.com
Anode 4Cl- -> 2Cl2.
Cathode 4H2O -> 2H2 + 4OH-.
2Cl2 +2H2O -> 3H+ + HOCl + OCl-.
HOCl + OCl- + uv light -> O2 + H+ + 2Cl-.
Following the process, we can see that there are 4H+ and 4OH- created, which nets out to pH neutral.
The chlorine gas generated is very acidic and creates 3 hydrogen ions for every 4 hydroxide ions created.
As the hypochlorous acid is broken down by UV, 1 more hydrogen ion is created for a net neutral result.
Assuming that chlorine gain and loss are equal, there's no pH rise.
However, there is hydroxide generated in the cell, which can cause scaling due to the production of calcium carbonate.
Ca2+ + HCO3- + OH- --> CaCO3 +H2O
If you have B(OH)3 (Boric acid) in the water, it buffers the pH change by taking some of the hydroxides as the pH rises.
Borates to contribute to the TA (Total Alkalinity), but their most important role is in providing Total Acidity, which is the resistance to pH rise from added base (Like hydroxide), whereas total alkalinity is the resistance to pH drop from added acid.
Ca2+ + HCO3- + OH- + B(OH)3 --> Ca2+ + HCO3- + B(OH)4-
Reducing the TA means that there is less Carbon dioxide, bicarbonate and carbonate in the water.
Carbon dioxide causes pH rise as it offgasses.
Bicarbonate and carbonate can create scale in the cell as it produces chlorine.
The general pool industry way overestimates the need to have TA as a buffer to pH change.


y = 100-(100/(1+10^(9.24– x))) and y = (100/(1+10^(9.24– x))) , x from 7to 11 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
Last edited:
- Sep 23, 2015
- 2,012
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V25
Pool shops I've been to have suggested a range of 125-150 ppm for TA. I've seen it mentioned a lot, on the internet, to keep the TA at 80-120 ppm.
This forum has suggested that there's nothing wrong with a TA of 60-70 pm for a SWG pool.
I actually suggested this a few months ago , to the pool testing man at the shop. My logic was that with going for a lower TA would require less Bicarb to be added. On top of this, my thought is that LESS acid would also be needed to bring the pH down. From what I read, the TA receives Hydrogen ions. This would mean if the pH needed to be reduced then any acid added would need to be " consumed? " by the TA before it had an effect on reducing pH. I know this flies in the face of advice from the pool shop man who said that a lower TA would mean MORE acid would be used ( I don't agree with this )
After reading the Orenda blogs I also learnt that a lower TA results in a lower pH "ceiling", another desirable outcome.
Is this reasoning valid? If I'm correct then is the only reasoning for shops to suggest a higher TA is to sell more chemicals?
Its best not to engage with the pool shop, they will drag you down to their level and beat you with experience.
A lower TA and higher pH target means less acid, less bicarb, a more stable pool and less profits for the pool shop. I’ve never added bicarb, there is enough in my tap water. During summer I use acid to reduce my TA.
Two things that may not have been covered yet is that from Wojtowics buffer intensity for both carbonate and cyanuric acid decrease with increasing pH. The carbonate buffer system is better at resisting a decrease in pH rather then an increase.
And although it’s hard to apply, the alkalinity of natural fresh water systems is around ~20ppm. And I just noticed this gem from the same article by Woltowitcs above where it is suggested that if dissolved CO2 and atmospheric CO2 where allowed to reach equilibrium an alkalinity of 4-17ppm would result.
In my opinion, the need for a buffer is largely overestimated.
No one is throwing a bunch of acid into the pool, so there is no need for any significant buffer.
Many sources say that the pH will swing wildly without a buffer, but it is just not true.
The pH moves for a reason.
If there is no reason, there is no movement.
A SWG pool can be managed with a TA that is low enough for the carbon dioxide to be in equilibrium with the air.
A trichlor pool does need the carbonate alkalinity to offset the acidity of the tabs, so it is useful and necessary for that purpose.
Using trichlor is adding acid to the pool, so it would need the buffer and the carbon dioxide also helps maintain the pH beyond just the buffering capacity.
If the pool surface is affected by the CSI, then the carbonate level has to be managed accordingly.
No one is throwing a bunch of acid into the pool, so there is no need for any significant buffer.
Many sources say that the pH will swing wildly without a buffer, but it is just not true.
The pH moves for a reason.
If there is no reason, there is no movement.
A SWG pool can be managed with a TA that is low enough for the carbon dioxide to be in equilibrium with the air.
A trichlor pool does need the carbonate alkalinity to offset the acidity of the tabs, so it is useful and necessary for that purpose.
Using trichlor is adding acid to the pool, so it would need the buffer and the carbon dioxide also helps maintain the pH beyond just the buffering capacity.
If the pool surface is affected by the CSI, then the carbonate level has to be managed accordingly.
Last edited:
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
In my opinion, the need for a buffer is largely overestimated.
No one is throwing a bunch of acid into the pool, so there is no need for any significant buffer.
Exactly. The only reason for pH-fluctuations is usually all the stuff the pool store asks us to dump into the pool. Don't throw random stuff in the pool, and guess what: pH is pretty stable.
SoDel
Well-known member
- May 27, 2022
- 515
- Pool Size
- 15000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Turbo Cell (T-CELL-5)
Extremely helpful info! I’ve always just accepted the conventional wisdom on alkalinity needs mainly because the fears created by the promise of “wild pH swings” being too difficult to monitor and verify (no hourly pH testing for me lol). If something were to somehow get in the pool that reduced pH (vinegar based sunscreen — who knows) I would consider it a good thing lol. I’ve kept TA around 50 which has meant no need for acid but for about once or twice monthly. Next season I’ll get it even lower.In my opinion, the need for a buffer is largely overestimated.
No one is throwing a bunch of acid into the pool, so there is no need for any significant buffer.
Many sources say that the pH will swing wildly without a buffer, but it is just not true.
The pH moves for a reason.
If there is no reason, there is no movement.
A SWG pool can be managed with a TA that is low enough for the carbon dioxide to be in equilibrium with the air.
A trichlor pool does need the carbonate alkalinity to offset the acidity of the tabs, so it is useful and necessary for that purpose.
Using trichlor is adding acid to the pool, so it would need the buffer and the carbon dioxide also helps maintain the pH beyond just the buffering capacity.
If the pool surface is affected by the CSI, then the carbonate level has to be managed accordingly.
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
Don't go too low, especially with a plaster pool. One issue is CSI. If water is very low in carbonates, it's near impossible to compensate with higher CH to keep CSI in a reasonable range.
And you do want some emergency protection. If someone accidentally added a gallon of MA instead of bleach (that happens, we had panicked reports like that here), then pH would crash if TA was for example only 30. And in a spa we are talking about little accidental spills, you add MA by the tea spoon there. Or an automated dispenser going rogue.
I wouldn't go below TA 50.
And you do want some emergency protection. If someone accidentally added a gallon of MA instead of bleach (that happens, we had panicked reports like that here), then pH would crash if TA was for example only 30. And in a spa we are talking about little accidental spills, you add MA by the tea spoon there. Or an automated dispenser going rogue.
I wouldn't go below TA 50.
- Sep 23, 2015
- 2,012
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V25
I keep running my numbers for CSI, a TA of 70ppm seems to be my happy place. At 70ppm TA with my pH drifting and manipulated between 7.7 and 8, my CSI is between -0.07 and -0.24.
A massive dump of rain water, snow melt or leaving the hose on will also tank your TA and pH.
A massive dump of rain water, snow melt or leaving the hose on will also tank your TA and pH.
SoDel
Well-known member
- May 27, 2022
- 515
- Pool Size
- 15000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Turbo Cell (T-CELL-5)
I have kind of opposite problem with CSI and pH. My pool wants to just raise pH to its Henry’s law ceiling, and fast. Long story but I had a local company, basically college kids doing a pool maintenance company, manage the pool for a year and developed a scale issue. So I try to keep CSI between -0.3 and -0.6 to avoid making scale any worse, especially in the very shallow areas that tend to get much hotter than the pool overall. With TA 50, CYA 50, CH 275, pH 8.1 (where pH has stabilized with the 50 TA, which tells me it’s more than 50, but test is +-10 at best so close enough), temp low to mid 80’s, it’s not quite there. Dropping TA below 50 but above 40 would be enough to do the trick and I think I could monitor where it was below 50 by watching pH, basically keep dosing acid as needed until pH stabilizes around 7.9 and TA test gives 40 or 50 as result. All a little moot for this season as the water gets colder, but next season . . . 
I know this is a bit off track, however..
If there’s a contaminant in the pool, we’re relying on the HOCL to oxidise it.
When an electron is lost from the contaminants molecule then this molecule then breaks down to a different molecule.
This continues.
Is this correct? Also, what is unique to the HOCL molecule that allows it to accept an electron from the contaminant?
If there’s a contaminant in the pool, we’re relying on the HOCL to oxidise it.
When an electron is lost from the contaminants molecule then this molecule then breaks down to a different molecule.
This continues.
Is this correct? Also, what is unique to the HOCL molecule that allows it to accept an electron from the contaminant?
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
Chlorine is in main-group 7 of the periodic table. That means it is only one electron short of becoming a main-group 8 element with a complete electron shell and achieving the ultimate goal of having the electron structure of a noble gas (Argon in this case). This makes Chlorine a very strong oxidizer, i.e. it likes to steal electrons from other stuff around. In the process, this stuff's "oxidation level" increases, because it's losing negatively charged electrons, i.e. the stuff is getting "oxidized". The Chlorine's oxidation level gets "reduced", it is gaining a negatively charged electron.
This works similarly with Bromine, which is also a main-group 7 element with a similar electron structure as Chlorine, just further "out", the inner shells are all filled. By gaining an electron, it reaches the noble-gas-like electron configuration of its neighbour Krypton in the periodic table. But let's stick with Chlorine.
The challenge is now to offer Chlorine in a form that is kind of stable in water, without losing it's oxidizing properties (you don't want the Chlorine to be to content with what it has where it is). That's where HOCl works well. And to a degree also OCl-, the extra electron sits with the Oxygen, and the Chlorine in OCl- still has oxidizing powers. But because HOCl is an electrically neutral molecule, it is easier to get through cell membranes and create havoc inside the cell:

This works similarly with Bromine, which is also a main-group 7 element with a similar electron structure as Chlorine, just further "out", the inner shells are all filled. By gaining an electron, it reaches the noble-gas-like electron configuration of its neighbour Krypton in the periodic table. But let's stick with Chlorine.
The challenge is now to offer Chlorine in a form that is kind of stable in water, without losing it's oxidizing properties (you don't want the Chlorine to be to content with what it has where it is). That's where HOCl works well. And to a degree also OCl-, the extra electron sits with the Oxygen, and the Chlorine in OCl- still has oxidizing powers. But because HOCl is an electrically neutral molecule, it is easier to get through cell membranes and create havoc inside the cell:

Last edited:
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.