- Jun 24, 2021
- 15,943
- Pool Size
- 29000
- Surface
- Vinyl
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60 Plus
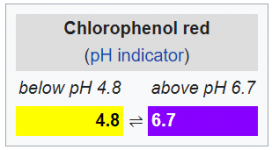
Odd situation. Did pH test was 7.4, within 2-3 seconds, the color went up rapidly ending with purple. Redid test 3 times. Meter said 7.4. Water temp 56.
Ideas?
Ideas?