so... I finally got around to install a SWG (basically same as circupool UL55). It has been running for 2 days and my PH goes up pretty fast since the installation. And by pretty fast I mean .6 in a day. The pool ph has a tendency to go up up but I don't remember ever that fast:
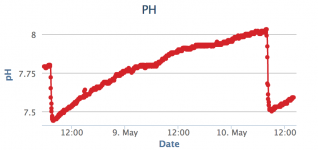
I figured I would test TA, last time I checked (about 4 months ago maybe), it was over 100 but PH was not going up this fast so I decided not to worry about it at the time (also confirmed by people here).
Well, TA is at 50 now and I did nothing to change it. I tested twice because I could not believe it.
Two questions:
1) if TA is at 50, why would PH rise up this much? Is that the SWG?
2) how did it get that low, as far as I understand there is a process that takes time to lower it on purpose... unless I tested it wrong before but I remember I did it a few times.
FC is at about 7 now
CYA is at 60 to 70
Calcium was at 350 last time, I have not checked it again yet

I figured I would test TA, last time I checked (about 4 months ago maybe), it was over 100 but PH was not going up this fast so I decided not to worry about it at the time (also confirmed by people here).
Well, TA is at 50 now and I did nothing to change it. I tested twice because I could not believe it.
Two questions:
1) if TA is at 50, why would PH rise up this much? Is that the SWG?
2) how did it get that low, as far as I understand there is a process that takes time to lower it on purpose... unless I tested it wrong before but I remember I did it a few times.
FC is at about 7 now
CYA is at 60 to 70
Calcium was at 350 last time, I have not checked it again yet




