Any effect of low CSI takes months or years to develop.
A pool is only closed for the winter for around 5 months. Any effect on the plaster by the CSI will only be for two or three months. And depending on the type of winter you get you may end up with high CSI or low CSI for a few weeks. You just don't know.
Thank you for that "months" or "years" length of time for a low CSI to aggressively "eat" the carbonates out of the plaster material.
Your number of "weeks" and "months" is the first time I've heard any length of time other than it takes a "long" time for aggressive water to suck something like fifty pounds of calcium
carbonate [edit: chloride] out of your plaster pool (I just recently added fifty pounds of calcium
carbonate [edit: chloride] for the winterization, so that's why I used that number).
I agree with you that the CSI is greatly affected by temperature which, if it fluctuates a lot during the winter, affects the CSI a lot during the winter.
But, when you eventually pour a hefty fifty pound bag of calcium
carbonate [edit: chloride] into the pool over a period of weeks, your body (while holding that bag in your hands) gets an instant up-front whole hardy healthy appreciation for how much cold aggressive water can leach out of the plaster over time!
If what you're advising the OP is a
temporary saturation imbalance is no big deal, I fully agree with you but temporary or not, the OP is asking about what happens when the TA is lowered (usually with muriatic acid), and what was well covered in the responses was how the pH is affected but
I just wanted to let the OP know that the CSI is also affected when you lower the (carbonate) TA.
That's all I wanted to add.
My suggestion to the OP is that when you lower the (carbonate) TA by x ppm, you (often) need to raise the CH by 3x or 4x that (all other things being equal).
Of course, as you noted, the water temperature matters greatly, but notice that winter is coming so colder water makes the need to raise CH even greater (all other things being equal).
- Lowering (carbonate) TA (in and of itself) lowers the CSI
- Lowering Temperature (in and of itself) lowers the CSI
I only want the OP to consider that both of those equilibria indicate that he may need to take a LOOK at the CSI as a result of those two conditions.
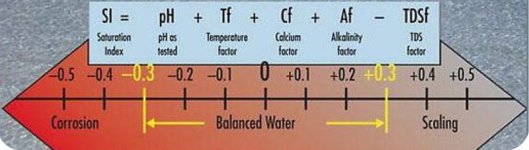
It is when CSI drops below -0.6 and really below -1.0 for a length of time that it should be a concern.
I agree the CSI can fluctuate wildly (mostly due to temperature swings).
I usually try to keep the CSI within 0.3 on each end of a balanced zero, mostly due to how much the CSI changes with the temperature alone.
It's when it goes negative that I worry far more than when it goes positive (although I don't have a SWCG pool where a positive CSI matters more).
That means I worry more about balance when the water is cold than when it's hot (but I don't have a SWCG).
In a closed pool for the winter pH will rise and water temperature will fall which offset each other for the CSI.
Hmmmm....... certainly in winter the water temperature will fall which tends to make the water balance more aggressive (lower CSI).
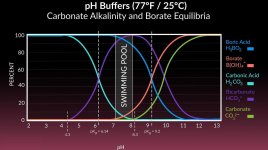
And certainly the pH is a complex equilibria that is controlled by a multitude of factors...
One of those factors is the amount of dissolved carbon dioxide in the water, which, I would think would go the OPPOSITE of what you are suggesting.
That is, wouldn't a lower temperature cause MORE carbon dioxide to stay dissolved in the colder water - which would tend to LOWER pH (not raise it)?
Of course, when pH is being discussed, everything affects everything else, so the carbonate alkalinity would ALSO go up in colder water, and that alone would maybe balance the pH rise due to the carbon dioxide also becoming more soluble in the colder water.
I do not know which temperature-related solubility equilibria wins as the water gets colder.
- The pH lowers due to the colder water being more soluble for carbon dioxide?
- Or, pH rises due to the colder water being more soluble for carbonates?
It's complicated, and I admit I do NOT fully understand how this all plays - but I do know what I'm asking the OP to consider which is two things that didn't seem to be covered in this thread, which I only want to bring to the fore for the OP to consider.
- Ask yourself WHY you're trying to lower TA (as the intended goal affects your calculations)
- Ask yourself what happens to the CSI as you lower TA (as you may need to compensate to maintain balance)