The TFP table for suggested parameters for my pool said

Using simple arithmetic, the midpoint between 7.2 and 8.0 would be 7.6
Allowing equal tolerance of 0.1 increments around that point, the ideal range would be 7.5-7.7, or 7.4-7.8 or similar
BUT….
the suggested ideal range is not around the midpoint. It is skewed towards the higher side. The midpoint of the ideal range is 7.7, which is not the midpoint between the minimum and maximum
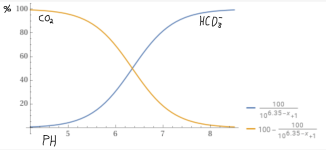
Is this because pH is a logarithmic scale, or is there some other reason?
Thanks,
Ken

Using simple arithmetic, the midpoint between 7.2 and 8.0 would be 7.6
Allowing equal tolerance of 0.1 increments around that point, the ideal range would be 7.5-7.7, or 7.4-7.8 or similar
BUT….
the suggested ideal range is not around the midpoint. It is skewed towards the higher side. The midpoint of the ideal range is 7.7, which is not the midpoint between the minimum and maximum
Is this because pH is a logarithmic scale, or is there some other reason?
Thanks,
Ken