Hi folks,
I know everyone here says to 'just use chlorine', when it comes to sanitisers, but nonetheless, I wonder if anyone can weigh in on this.
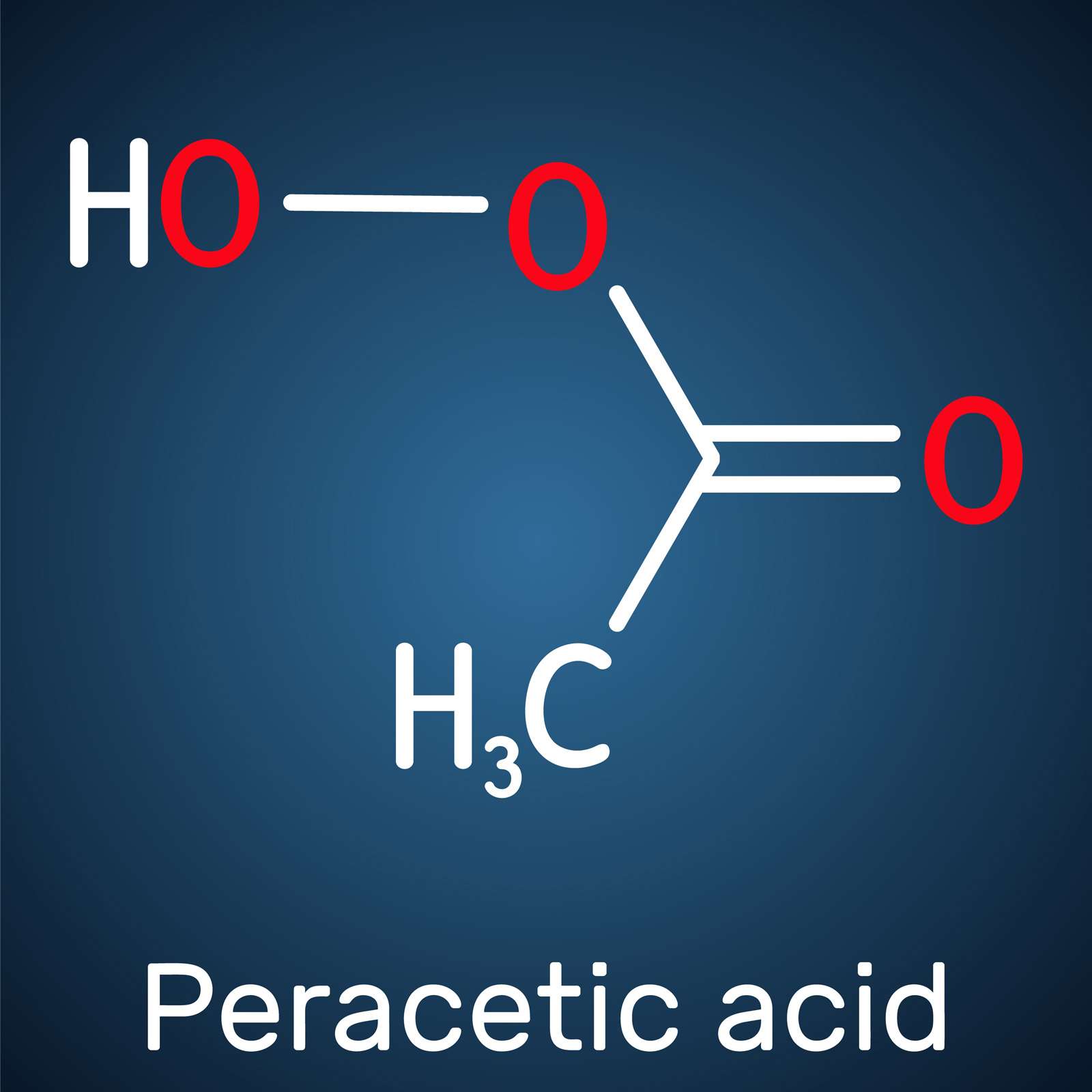
Peracetic acid (PAA) - made from mixing concentrated hydrogen peroxide (h2o2) with acetic acid (vinegar), and using an acid catalyst to kickstart the reaction - is apparently a strong chlorine alternative for municipal water treatment. (Seems to be raved about in that industry.)
I wonder of its usefulness for pools?
It seems to be stronger than chlorine as a disinfectant in many cases, it can be used as a steriliser (unlike chlorine), and it doesn't form disinfection byproducts (DPBs) like chlorine can (at least if chlorine's not done properly).
AFAIK, PAA doesn't last very long - when added to regular water it breaks down in under an hour, or even in mere minutes. It does seem to leave residual h2o2 (and also PAA is always sold in solutions which have a certain degree of h2o2 in it alongside the PAA), but I know that h2o2 is more of an oxidiser rather than a good sanitiser (microbe killer), and h2o2 can eat away at chlorine, so the h2o2 isn't a benefit in most cases.
But could a PAA 'zap' of a pool - including swishing water around with the pool broom to make it react with the whole pool depth rather than just the top - help reduce chlorine usage overall, if it means less surviving microbes are needing to be handled by the chlorine?
(I like how borates can lessen the amount of chlorine needed by stabilising pH and also by acting as a mild algaestat. Could PAA similarly increase chlorine's effectiveness, because of how strong it is?)
As for algae, I have concerns / am confused. I see scientific discourse that seems to suggest that PAA can actually serve as useful fuel to help algae grow, not kill it. If true, it'd only be advisable when pool is already crystal clear and no nascent, cloudy / mature algae present. Perhaps dosage matters - higher PAA concentration harms algae and lower helps it.
About residuality, I'm confused as to why PAA is so useful in pre-treating city water, if it barely lasts for long in the water - is it a quick but extremely effective 'zap', as I'm suspecting? And does the residual acetic acid (the other byproduct) break down later in the stream? (If not, it would taste and smell a bit vinegary right?)
Lastly I wonder if PAA has usefulness for 'spot cleaning' or helping to rid biofilm from plumbing lines. I know chlorine shocking can do everything, but it's interesting to think of alternatives out there. Doing a supershock for days on end because of a small area seems exhausting and maybe not always the most economical choice.
What I do know is that peracetic acid is hazardous stuff to handle. PPE is needed (gloves, eye protection, clothes) - don't breathe in, preferably decanter it outside, don't spill on skin, and don't get in eyes.
Any benefits to it?
Thanks,
fresh
P.S. How's this for diving into the deep end?
I know everyone here says to 'just use chlorine', when it comes to sanitisers, but nonetheless, I wonder if anyone can weigh in on this.
Peracetic acid (PAA) - made from mixing concentrated hydrogen peroxide (h2o2) with acetic acid (vinegar), and using an acid catalyst to kickstart the reaction - is apparently a strong chlorine alternative for municipal water treatment. (Seems to be raved about in that industry.)
I wonder of its usefulness for pools?
It seems to be stronger than chlorine as a disinfectant in many cases, it can be used as a steriliser (unlike chlorine), and it doesn't form disinfection byproducts (DPBs) like chlorine can (at least if chlorine's not done properly).
AFAIK, PAA doesn't last very long - when added to regular water it breaks down in under an hour, or even in mere minutes. It does seem to leave residual h2o2 (and also PAA is always sold in solutions which have a certain degree of h2o2 in it alongside the PAA), but I know that h2o2 is more of an oxidiser rather than a good sanitiser (microbe killer), and h2o2 can eat away at chlorine, so the h2o2 isn't a benefit in most cases.
But could a PAA 'zap' of a pool - including swishing water around with the pool broom to make it react with the whole pool depth rather than just the top - help reduce chlorine usage overall, if it means less surviving microbes are needing to be handled by the chlorine?
(I like how borates can lessen the amount of chlorine needed by stabilising pH and also by acting as a mild algaestat. Could PAA similarly increase chlorine's effectiveness, because of how strong it is?)
As for algae, I have concerns / am confused. I see scientific discourse that seems to suggest that PAA can actually serve as useful fuel to help algae grow, not kill it. If true, it'd only be advisable when pool is already crystal clear and no nascent, cloudy / mature algae present. Perhaps dosage matters - higher PAA concentration harms algae and lower helps it.
About residuality, I'm confused as to why PAA is so useful in pre-treating city water, if it barely lasts for long in the water - is it a quick but extremely effective 'zap', as I'm suspecting? And does the residual acetic acid (the other byproduct) break down later in the stream? (If not, it would taste and smell a bit vinegary right?)
Lastly I wonder if PAA has usefulness for 'spot cleaning' or helping to rid biofilm from plumbing lines. I know chlorine shocking can do everything, but it's interesting to think of alternatives out there. Doing a supershock for days on end because of a small area seems exhausting and maybe not always the most economical choice.
What I do know is that peracetic acid is hazardous stuff to handle. PPE is needed (gloves, eye protection, clothes) - don't breathe in, preferably decanter it outside, don't spill on skin, and don't get in eyes.
Any benefits to it?
Thanks,
fresh
P.S. How's this for diving into the deep end?