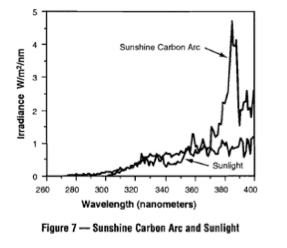
The test is referred to as an “accelerated” test, which means that it is somehow faster than a normal “leave it outside for years” test.
You would need to read the actual test report for the details of what intensity of light was used and what the assumptions and conclusions were.
It might have been done at 1 x Sun or 2 X or 3 X.
 www.micomlab.com
www.micomlab.com

 www.micomlab.com
www.micomlab.com

 www.micomlab.com
www.micomlab.com
You would need to read the actual test report for the details of what intensity of light was used and what the assumptions and conclusions were.
It might have been done at 1 x Sun or 2 X or 3 X.
ASTM G155 is used to perform accelerated ageing on a wide range of products and industries including products for the automotive industry, surface coatings, pharmaceutical light stability tests, printing inks, roofing, rubber, adhesives, textiles, geotextiles and many others.

UV Testing FAQs - 15 most frequent questions and answers - Micom
UV Testing FAQs section assembles the most frequently asked questions we receive from clients and companies regarding different UV tests.

Weather-Ometer®️* testing services available at Micom Laboratories
Weather-Ometer®️* testing is a UV aging technique used to simulate sun exposure on coatings. Learn more about the weatherometer testing and our numerous other tests.

Accelerated Aging Testing Services | Micom Laboratories
Accelerated aging in laboratories to test how materials and products could react to adverse conditions encountered in the real world. Learn more.

