I probably need to concentrate more on keeping the TA
With a SWG, I'd always say "go for it", as borates really help to keep the cell clean and extend it's lifetime. But with liquid chlorine, I'd recommend to first explore how far you can get with proper TA management.
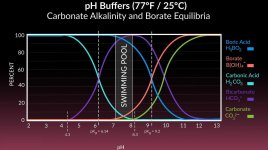
To give you an idea, how far out of equilibrium a pool at "traditional" TA and pH recommendations is, I ran a few calcs (with chem geek's spreadsheet). This chart shows the concentration of dissolved CO₂ (in absolute values measured in mol/l, not as a relative percentage of the total amount of carbonates as in the chart you have shown, so you can see how the amount of CO₂ rises with higher TA):
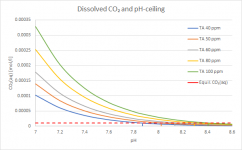
The red line shows the CO₂ concentration where the dissolved CO₂ is in equilibrium with atmospheric CO₂. You can see how far away from that equilibrium you are at for example TA=100ppm and pH=7.2, a "well recommended" place to be by the traditional pool industry. The further away from that red line you are, the faster CO₂ will outgas to reach equilibrium. This really shows how the cat is chasing its tail by trying to maintain this parameter combination. These curves are calculated for 25°C (77°F), CYA 50ppm, Salt 1000ppm and no borates.
To see the places where you'd actually rather be, here a zoom-in:
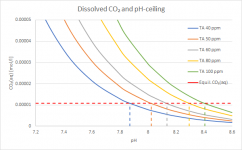
Now you can see a bit better at which pH you reach the "ceiling" depending on TA. This is basically the tail end of the yellow curve in your chart, but multiplied with the total amount of carbonates in all its possible forms, hence the splitting of the one curve into different curves for each TA. The quantitative details change of course depending on parameters like temperature and especially CYA - maintaining the same TA at higher CYA means less Carbonate Alkalinity, but the general picture doesn't change. The lower the TA, the sooner CO₂ will stop outgassing and the pH-rise will slow down. Don't take the shown absolute values to serious, I just want to show the principle.
One has to find the sweetest spot possible under the personal circumstances. In areas with high evaporation with constant additions of high TA fill water, it is a constant process to maintain lowish TA. Someone with a vinyl liner can afford a lower TA than someone with a plaster pool who has to watch not just high, but also low CSI.
You don't want to go too low, even though that might look tempting looking at the pH-ceiling. TFP doesn't recommend to go lower than TA 50ppm, or you'll risk that your next MA addition might crash your pH. Take PoolMath's warning in the "Effects of adding" serious that pH calculations "will be approximately correct" for standard water parameters. I wouldn't trust the calculated amount of MA required for a certain pH change when TA is only 30, for example.
And be careful with things like increasing CH to compensate a lower TA in regards to CSI before you understand the long-term CH development in your pool. It's very easy to increase CH, but not that easy to get rid of it again. Rather than aiming straight away for what you might now consider the "perfect" water parameters, give it some time to understand how your pool behaves and how TA and CH develop over time before making changes that would require a drain-refill to undo them. Even relatively low CH fill water adds up over time when evaporation losses are high. But depending on rain in the colder months, a CH rise over summer might get compensated on a yearly level.
Take your time, understand your pool. Rome wasn't built in a day.
And regarding your question
What would make the TA change over time (once I have it perfectly balanced)?
Don't worry - if your fill water has high TA, then this will probably never happen. Most get to a situation where their pool settles at a TA where the rise due to fill water gets compensated by (ideally not that frequent) MA additions. Some are lucky and get to a rock solid TA and pH. High TA fill water additions drive TA up, a lot of rain with overflow can reduce TA. Acid additions reduce TA - and this includes acidic sources of chlorine like Trichlor. Apart from draining-refilling to get rid of CYA, chlorination with Trichlor also requires regular additions of baking soda to stop pH and TA from crashing - that's where the traditional high TA recommendations come from.
This all drifted a bit away from the initial question on how to add boric acid, but I hope it helps a bit on the questions of when and why to add boric acid.