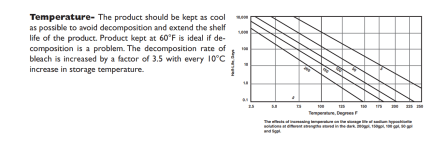
Hey all,
I spend most of my time on the spa side of the forum but am putting together a proposal to add a new heater to our 60000 gallon gunite community pool in southeast Michigan. Our current heater can only get it to 82 and we are looking for more like 85 or so.. Those in opposition are going to take any angle they can to block the proposal and one thing that's come up is increased consumption of sanitizer etc. Can anyone speak to any estimate of what kind of increase in consumption we are looking at with an additional bump of only 4 degrees or so? I understand algae and bacteria can proliferate more rapidly in warmer water but wouldn't expect a drastic change with a 4 degree bump.
We have a commercial pool company that takes care of the pool daily but I've been less than impressed with their answers about other issues and would love to have some background before speaking to them more on Monday.
As far as I know the commercial pool requirements for FC don't change when jumping from 82 to 85.
I spend most of my time on the spa side of the forum but am putting together a proposal to add a new heater to our 60000 gallon gunite community pool in southeast Michigan. Our current heater can only get it to 82 and we are looking for more like 85 or so.. Those in opposition are going to take any angle they can to block the proposal and one thing that's come up is increased consumption of sanitizer etc. Can anyone speak to any estimate of what kind of increase in consumption we are looking at with an additional bump of only 4 degrees or so? I understand algae and bacteria can proliferate more rapidly in warmer water but wouldn't expect a drastic change with a 4 degree bump.
We have a commercial pool company that takes care of the pool daily but I've been less than impressed with their answers about other issues and would love to have some background before speaking to them more on Monday.
As far as I know the commercial pool requirements for FC don't change when jumping from 82 to 85.