I'm preparing to have new plaster installed so I have been researching it.
I tested my water at the hose and it's:
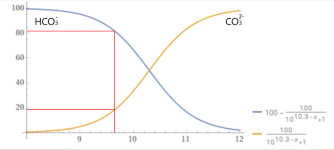
PH: 9.65
TA: 70
CH: 40
My understanding is that this water would be extremely aggressive towards new paster. So I want to use the bicarb startup method advocated here.
However all of the examples I've seen assume combined TA and CH of the fill water start off around 200 to 250ppm. Mine is only 110ppm combined.
So I'm wondering if my fill water is just too far out of spec for the bicarb method to be utilized. If that's the case I'm guessing my only other option would be to have pretreated water trucked in.
Thank you for your time,
Joseph
I tested my water at the hose and it's:
PH: 9.65
TA: 70
CH: 40
My understanding is that this water would be extremely aggressive towards new paster. So I want to use the bicarb startup method advocated here.
However all of the examples I've seen assume combined TA and CH of the fill water start off around 200 to 250ppm. Mine is only 110ppm combined.
So I'm wondering if my fill water is just too far out of spec for the bicarb method to be utilized. If that's the case I'm guessing my only other option would be to have pretreated water trucked in.
Thank you for your time,
Joseph