a Q about phospates
- Thread starter DMS2014
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
His hypothesis is that orthophosphates attach to the metal plates and cause an interference with the flow of electrons between the anode and the cathode.It appears that since orthophosphates attach to metals they attach to the anode and cause an interference with the flow of electrons between the anode and the cathode of the salt chlorine generator.
It's nothing more than pure speculation with zero evidence that it actually happens.
He is speculating about the interference and about the cause of any hypothetical interference.
So, it is a hypothesis about the cause of a hypothetical problem.
Maybe he should actually test the first hypothesis (Interference) before trying to hypothesize about what might be causing the hypothetical interference.
Also, the hypothesis about the mechanism of interference is weak and ridiculously vague.
Why would phosphates only attach to the anode and not the cathode?
Also, electrons do not flow between the anode and cathode.
Electrons flow from the cathode to hydrogen ions and from chloride ions to the anode.
Anode 4Cl- -> 2Cl2.
Cathode 4H2O -> 2H2 + 4OH-.
2Cl2 +2H2O -> 3H+ + HOCl + OCl-.
HOCl + OCl- + uv light -> O2 + H+ + 2Cl-.
Following the process, we can see that there are 4H+ and 4OH- created, which nets out to pH neutral.
The chlorine gas generated is very acidic and creates 3 hydrogen ions for every 4 hydroxide ions created.
As the hypochlorous acid is broken down by UV, 1 more hydrogen ion is created for a net neutral result.
Assuming that chlorine gain and loss are equal, there's no pH rise.
Last edited:
In any case, I have already shown that many SWGs will give you an indication of poor performance.
In my opinion, all SWGs should be able to measure actual performance vs. factory specifications for performance.
This is how Hayward SWGs work and you can get the performance of the cell quite easily by dividing the reported instant salinity by the actual salinity.
All SWGs can and should do the same calculations.
The Intellichlor can use its conductivity meter and compare it to a formula for salinity (Like the Hayward SWGs) and it can continuously report the cell performance.
In my opinion, all SWGs should be able to measure actual performance vs. factory specifications for performance.
This is how Hayward SWGs work and you can get the performance of the cell quite easily by dividing the reported instant salinity by the actual salinity.
All SWGs can and should do the same calculations.
The Intellichlor can use its conductivity meter and compare it to a formula for salinity (Like the Hayward SWGs) and it can continuously report the cell performance.
Antlo
Well-known member
Someone asked for a link to studies and the link given was to a magazine article. It seemed like the person had confused a magazine article with a scientific study that showed a relationship. Not really interested in scrolling all the way back through the replies though, hope you figure out the issue if you're OP!Did anyone in this forum or in the original article actually make the claim that it was true?
- May 3, 2007
- 18,073
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
I am the one that posted the article and no I did not mistake it for scientific fact. I merely suggested that the premise was plausible. You might want to reread the thread again.Someone asked for a link to studies and the link given was to a magazine article. It seemed like the person had confused a magazine article with a scientific study that showed a relationship. Not really interested in scrolling all the way back through the replies though, hope you figure out the issue if you're OP!
a Q about phospates
I did a search and read a 2011 post about phos in someone's pool BUT Husband was wondering if that could have anything to do with our constant high pH issues. Our FC has been stable all week at 5.5. our SWG is at 70% which I feel is high for this time of year Did a full test today and results...
I did a search and read a 2011 post about phos in someone's pool BUT Husband was wondering if that could have anything to do with our constant high pH issues.
To be clear, the main question was about the phosphates possibly causing the pH to be high.Our FC has been stable all week at 5.5. our SWG is at 70% which I feel is high for this time of year
The answer to that is a definite no.
The second issue raised was that the SWG output seemed to be higher than expected to maintain the chlorine.
The performance of the cell can be verified by dividing the instant salinity by the actual salinity.
Anything in the water consuming chlorine can be ruled in or out by an OCLT.
Overnight Chlorine Loss Test
So trying to jump back to the OP and return this thread to the original topic……I did a search and read a 2011 post about phos in someone's pool BUT Husband was wondering if that could have anything to do with our constant high pH issues. Our FC has been stable all week at 5.5. our SWG is at 70% which I feel is high for this time of year
Did a full test today and results were:
FC 5.5
CH 375 (amazingly and strangely down from the 450 it's been at for two years)
pH was 8.2
TA 70
CYA 50
Salt 3200
WENT to Leslie's last week bc I couldn't believe that with two socks of CYA it was still not registering beyond 20ish (chem new) . it was accurate. and ,shockingly, they were spot on with most of my tests from last week. They said my Phos was 655 and the ideal range is 0-100. They said my salt was 3111.
You feel running the SWG at 70% is high for this time of the year, have you by any chance tried an OCLT?
- May 3, 2007
- 18,073
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
Any PH difference near the plate might have an effect although I would think it would be the cathode.Why would phosphates only attach to the anode and not the cathode?
I would agree that over time, the net reaction is neutral but only after accounting for all the reactions some being non-local to the anode and cathode.Also, electrons do not flow between the anode and cathode.
Electrons flow from the cathode to hydrogen ions and from chloride ions to the anode.
Anode 4Cl- -> 2Cl2.
Cathode 4H2O -> 2H2 + 4OH-.
2Cl2 +2H2O -> 3H+ + HOCl + OCl-.
HOCl + OCl- + uv light -> O2 + H+ + 2Cl-.
Following the process, we can see that there are 4H+ and 4OH- created, which nets out to pH neutral.
Where and when does this reaction actually occur?
2Cl2 +2H2O -> 3H+ + HOCl + OCl-
If downstream from the plates, then near the cathode, the PH could be slightly higher than the rest of the water due to the OH-.
When I took apart my old cell, scale most certainly collects on one side of the plates vs the other so there is definitely a bias although I did not take note of which was the anode and which was the cathode but I should be able to do that for the next cell.


One side clearly has more scale than the other on many of the plates.
Chlorine gas dissolves pretty fast, but I do not know exactly how fast.Where and when does this reaction actually occur?
2Cl2 +2H2O -> 3H+ + HOCl + OCl-
Most cells are self cleaning and reverse polarity every cycle, so there is no permanent anode or cathode.
Each side of the plate should spend roughly the same amount of time being an anode and being a cathode.
SWG (new 2020) T-15 40k
The cell is 4 years old.
If it is underperforming, that can be explained by the age.
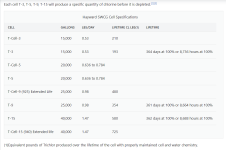
Hayward SWCG Cell Specifications
(1)Equivalent pounds of Trichlor produced over the lifetime of the cell with properly maintained cell and water chemistry.
The cell is 4 years old.
If it is underperforming, that can be explained by the age.

Aquarite T-Cells
Cell chlorine Generating Capacity
Each cell T-3, T-5, T-9, T-15 will produce a specific quantity of chlorine before it is depleted.Hayward SWCG Cell Specifications
| CELL | GALLONS | LBS/DAY | LIFETIME CL LBS(1) | LIFETIME |
|---|---|---|---|---|
| T-Cell-3 | 15,000 | 0.53 | 210 | |
| T-3 | 15,000 | 0.53 | 193 | 364 days at 100% or 8,736 hours at 100% |
| T-Cell-5 | 20,000 | 0.636 to 0.784 | ||
| T-5 | 20,000 | 0.636 to 0.784 | ||
| T-Cell-9 (925) Extended Life | 25,000 | 0.98 | 480 | |
| T-9 | 25,000 | 0.98 | 354 | 361 days at 100% or 8,664 hours at 100% |
| T-15 | 40,000 | 1.47 | 580 | 362 days at 100% or 8,688 hours at 100% |
| T-Cell-15 (940) Extended life | 40,000 | 1.47 | 725 |
The first post indicates low CYA, which can account for higher FC losses.WENT to Leslie's last week bc I couldn't believe that with two socks of CYA it was still not registering beyond 20ish (chem new) .
Pool Care Basics
Overnight Chlorine Loss Test
SLAM Process
PoolMath
FC/CYA Levels
Test Kits Compared
scale most certainly collects on one side of the plates vs the other
Any type of scale can reduce the performance of the cell.One side clearly has more scale than the other on many of the plates.
I think that that is a well established fact.
Scale prevents water from contacting the plates and creates a barrier (insulation) to the flow of electrons.
You can get calcium carbonate and/or calcium phosphate scale as well as other types of scale.
The reported level for phosphate is less than 1 ppm, so probably not a high risk of scale.
Scale is usually visible and the cell can certainly be checked to make sure that it is clean.
If, and only if, the cell is scaled, then clean the cell as needed.
Calcium carbonate dissolves with acid.
Calcium phosphate does not dissolve so easily.
- May 3, 2007
- 18,073
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
But that reaction would be close to the anode rather than the cathode because that is where the Cl2 is. Flow near walls is laminar so does not tend to mix easily with flow away from the wall. So the two reactions may actually be separated for some time with the PH on the cathode increased.Chlorine gas dissolves pretty fast, but I do not know exactly how fast.
I knew better. Completely forgot about that. But then it brings up new questions about the asymmetry in scale. probably has to do with the fact that the outer 2 plates and the inner plate are the only ones with voltage.Most cells are self cleaning and reverse polarity every cycle, so there is no permanent anode or cathode.
Each side of the plate should spend roughly the same amount of time being an anode and being a cathode.
This is exactly the line of reasoning I outlined earlier and why I though the phosphates could impact performance as well as cell lifetime. And yes, for the OP, I agree, probably not an issue for the reported PH/TA/CH. It would require something around 5 ppm to start to precipitate. However, with the reported CH of 375, PH of 9.5 and water temp of 90F, it is possible even with 1 ppm. We still don't know for sure if the cathode plate does not have very high PH for the reasons I explained above. One could possibly estimate the PH rise if the flows remained separated for the entire length of the cell based upon flow rate and current. The only issue is taking into account all of the side reactions.Any type of scale can reduce the performance of the cell.
I think that that is a well established fact.
Scale prevents water from contacting the plates and creates a barrier (insulation) to the flow of electrons.
You can get calcium carbonate and/or calcium phosphate scale as well as other types of scale.
The reported level for phosphate is less than 1 ppm, so probably not a high risk of scale.
Scale is usually visible and the cell can certainly be checked to make sure that it is clean.
If, and only if, the cell is scaled, then clean the cell as needed.
Calcium carbonate dissolves with acid.
Calcium phosphate does not dissolve so easily.
I get scale on my SWG even when CSI of the main water body is less than zero so I am fairly confident the PH near the plates must be higher than the rest of the water. Just not easily measured.
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

