Disconnect the bond wire from the pump and measure for AC and DC voltage between the bond lug and the bond wire.
A bonding question I can't find the answer to
- Thread starter Towebud
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Just because a magnet didn’t stick to it doesn’t mean it’s aluminum. In a motor casing application your absolutely wouldn’t use a ferromagnetic steel because of the possible induction effects on the motor windings or field effects caused by a soft magnetic material. There are grades of steel that are completely non-magnetic.
But I agree, contact the motor manufacturer as that would be your quickest way of finding out how the casing is constructed.
Galvanic corrosion is going to be tricky to track down because there may not be any measurable voltages until things get wet. So it may be a transient phenomenon that takes rain and environmental moisture to make it happen. I agree that aluminum can “rust” but it’s only under extreme situations for it to happen chemically (pH below 4 or above 10). Chlorides and sulfates accelerate aluminum corrosion but that would be pretty evident with a pump leak.
But I agree, contact the motor manufacturer as that would be your quickest way of finding out how the casing is constructed.
Galvanic corrosion is going to be tricky to track down because there may not be any measurable voltages until things get wet. So it may be a transient phenomenon that takes rain and environmental moisture to make it happen. I agree that aluminum can “rust” but it’s only under extreme situations for it to happen chemically (pH below 4 or above 10). Chlorides and sulfates accelerate aluminum corrosion but that would be pretty evident with a pump leak.
Looks like aluminium to me.
Steel would be brown.
Aluminum can corrode quite rapidly in certain cases.
The aluminum acts as a sacrificial anode to everything on the bonding grid.



Steel would be brown.
Aluminum can corrode quite rapidly in certain cases.
The aluminum acts as a sacrificial anode to everything on the bonding grid.

- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
It’s possible that it’s aluminum. The thing to understand about zinc “white rust” is that there is almost a 100x change in volume when the zinc goes from zinc metal to zinc carbonate/oxide/hydroxide (it’s a mixed anion system). So the zinc metal creates lots of powdery white rust which is why the exterior paint swells and bulges so much. If you wire brush off all the white rust down to the fresh zinc layer, it will look brown/dark-grey, not metallic, due to the nature of zinc surface (micro porosity). Once the zinc is fully consumed, you can see what the base metal layer is. Normally, before applying zinc galvanization, one should either use a phosphate or chromate conversion coating on any ferrous alloys to seal the surface and keep out oxygen which initiates the iron oxidation process.
Aluminum oxidation doesn’t produce as much volume as zinc but it can look similar in texture. With aluminum metal, you want to paint (cheap) or powder coat (more reliable) the outer surface to ensure good sealing. Unfortunately, bolts and threaded connection points, like the bonding lug, can cause damage to the outer layer and that is usually where corrosion will start.
Whatever the underlying metal is, Pentair has a problem with these pumps. It seems that moist environments really destroys the outer casings on these motors making them look very ugly and ancient. They need to take this up with their electric motor supplier to see what can be done about it. Improper selection of paint on zinc (or aluminum) can actual lead to early failures. Some paints contain chlorides or sulfates as part of their emulsions and that would definitely kill these casings. Perhaps the supplier decided to use a cheaper paint on these to save a few pennies per motor … when manufacturing in high volumes, it’s not uncommon to make minor changes to say money and boost profit. Happens all the time.
Aluminum oxidation doesn’t produce as much volume as zinc but it can look similar in texture. With aluminum metal, you want to paint (cheap) or powder coat (more reliable) the outer surface to ensure good sealing. Unfortunately, bolts and threaded connection points, like the bonding lug, can cause damage to the outer layer and that is usually where corrosion will start.
Whatever the underlying metal is, Pentair has a problem with these pumps. It seems that moist environments really destroys the outer casings on these motors making them look very ugly and ancient. They need to take this up with their electric motor supplier to see what can be done about it. Improper selection of paint on zinc (or aluminum) can actual lead to early failures. Some paints contain chlorides or sulfates as part of their emulsions and that would definitely kill these casings. Perhaps the supplier decided to use a cheaper paint on these to save a few pennies per motor … when manufacturing in high volumes, it’s not uncommon to make minor changes to say money and boost profit. Happens all the time.
I'm 99.999% sure that it is aluminum.
The magnetic fields probably induce currents in the aluminum.
The aluminum is an anode to everything on the bonding grid.
Aluminum cup anchors will usually corrode pretty badly in a few years.
The surface of the aluminum might be protected by the aluminum oxide passivation layer, but voltage causing a loss of electrons goes into the atomic metal and causes oxidation below the surface.
The magnetic fields probably induce currents in the aluminum.
The aluminum is an anode to everything on the bonding grid.
Aluminum cup anchors will usually corrode pretty badly in a few years.
The surface of the aluminum might be protected by the aluminum oxide passivation layer, but voltage causing a loss of electrons goes into the atomic metal and causes oxidation below the surface.
Flboy44
Well-known member
FYI, I’m just outside of Orlando, north west.
Something to consider. Because of our ground being mostly sand, achieving a solid ground can be an issue! After my house suffered two lightning strikes, I installed a grounding grid! My house is all metal studs and was building up a charge!
Every 6 feet, I drove a copper rod and tied them all together! If your house was ever struck, even indirectly, the sand around your grounding rod may have glassified!
FYI, new rods, in Florida, trick to install? A heavy hammer drill and a ladder! A few minutes each!
Something to consider. Because of our ground being mostly sand, achieving a solid ground can be an issue! After my house suffered two lightning strikes, I installed a grounding grid! My house is all metal studs and was building up a charge!
Every 6 feet, I drove a copper rod and tied them all together! If your house was ever struck, even indirectly, the sand around your grounding rod may have glassified!
FYI, new rods, in Florida, trick to install? A heavy hammer drill and a ladder! A few minutes each!
Both metals look almost exactly the same.Aluminum cup anchors will usually corrode pretty badly in a few years.
Below is an aluminum cup anchor showing typical corrosion.

- May 3, 2007
- 18,068
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
I would think that just keeping the pump motor dry would prevent the corrosion on the motor.
Keeping the motor dry would help, but I do not think that it will completely solve the problem.
In my opinion, there is something in the environment that is causing excessive corrosion.
Probably some sort of stray current.
Aluminum is a poor choice for the motor casing.
I would use a good stainless steel, brass, titanium, platinum, ruthenium or indium for the motor casing.
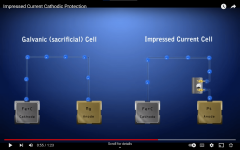
I think that a large zinc anode with an impressed current would probably help until the problem can be found.
In my opinion, there is something in the environment that is causing excessive corrosion.
Probably some sort of stray current.
Aluminum is a poor choice for the motor casing.
I would use a good stainless steel, brass, titanium, platinum, ruthenium or indium for the motor casing.
I think that a large zinc anode with an impressed current would probably help until the problem can be found.
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Aluminum is a poor choice for the motor casing.
I would use a good stainless steel, brass, titanium, platinum, ruthenium or indium for the motor casing.
Agreed ... but no one will want to pay $250,000 for a pump with a platinum motor casing nor hire a 24/7 security detail to guard it against theft. And some forms of ruthenium oxide are volatile gases at standard temperatures and pressure so the pump casing would simply evaporate away as it corrodes ....
I think that a large zinc anode with an impressed current would probably help until the problem can be found.
This may or may not work. The implementation is key as you need to have the zinc anode properly bagged and kept moist in order to ensure that it dissolves uniformly. If you bury zinc in soil without the proper environment surrounding it, it will simply form a passive oxide layer that is non-conductive and the impressed current flow will stop. The aluminum case will then start to corrode all over again.
I think Mark is on the right track - the motor needs to be taken out of the open environment and covered or shielded from getting wet. Without moisture, the metal will have a much harder time corroding as there is not enough ambient oxygen in the air to cause much corrosion.
Last edited:
Assuming about 750 grams of platinum, the cost is only about $24,677.24.No one will want to pay $250,000 for a pump with a platinum motor casing nor hire a 24/7 security detail to guard it against theft.
If you want to solve the problem, you can’t be a cheapskate.
I don’t think so.The implementation is key as you need to have the zinc anode properly bagged and kept moist in order to ensure that it dissolves uniformly. If you bury zinc in soil without the proper environment surrounding it, it will simply form a passive oxide layer that is non-conductive and the impressed current flow will stop.
Why does the aluminum casing corrode when it is just sitting outside in the air?
If you got a 10 lb zinc anode and increased the voltage between the zinc and the bond wire by about 1.5 volts, the zinc should corrode preferentially to the aluminum.
You could probably just leave the zinc out on the ground and rinse it off periodically to keep it active or turn up the voltage.
You could also connect a magnesium anode as they are even more active than zinc.
If you bury the anode in the ground deep enough where it is wet, that should be good enough, especially if you increase the voltage.

Last edited:
Maybe create an airtight housing around the pump and fill it with inert nitrogen.Without moisture, the metal will have a much harder time corroding as there is not enough ambient oxygen in the air to cause much corrosion.
- May 3, 2007
- 18,068
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
Galvanic corrosion only occurs in the presence of an electrolyte solution. Remove the water, there is no corrosion. Of course that is easier said than done and not always possible.
This is more of a problem in very wet locations such as the OPs (Orlando) or near the ocean with high humidity. The copper wire connection directly to the motor housing could be the source. Especially since it penetrates the paint at that location. It is fairly easy for both of those to get wet at the same time as they are in close proximity to each other and lower on the motor where more water will hit it. It just needs a thin film. Keeping both of these dry would certainly help. I also wonder if they had moved it higher on the motor body, it would have caused less of an issue. The bonding point for my VS pump motor is on the back and top of the motor almost underneath the drive so I would expect that location to be more protected from moisture than a location such as that the Intelliflo uses.
But I agree that the rate of corrosion seems very high pointing to another factor. NEV is another source that could be accelerating the corrosion although AC electrolytic reactions are much less than DC reactions (reason AC is not used for SWGs) but it still might have an impact.
This is more of a problem in very wet locations such as the OPs (Orlando) or near the ocean with high humidity. The copper wire connection directly to the motor housing could be the source. Especially since it penetrates the paint at that location. It is fairly easy for both of those to get wet at the same time as they are in close proximity to each other and lower on the motor where more water will hit it. It just needs a thin film. Keeping both of these dry would certainly help. I also wonder if they had moved it higher on the motor body, it would have caused less of an issue. The bonding point for my VS pump motor is on the back and top of the motor almost underneath the drive so I would expect that location to be more protected from moisture than a location such as that the Intelliflo uses.
But I agree that the rate of corrosion seems very high pointing to another factor. NEV is another source that could be accelerating the corrosion although AC electrolytic reactions are much less than DC reactions (reason AC is not used for SWGs) but it still might have an impact.
- Aug 20, 2020
- 7,764
- Pool Size
- 27000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60
Would be very curious if the problem goes away after switching to a different model pump. Seems to be this pump is prone to that kind of thing. So maybe that’s a cheaper option than platinum cases. 
I have solar panels, so maybe an additional connection to ground. I'm not sure what's behind it. I did test for an electrical current, and there is none.What is behind this plate?

Would it help to cover that connection with dielectic grease, so it won't get wet?Galvanic corrosion only occurs in the presence of an electrolyte solution. Remove the water, there is no corrosion. Of course that is easier said than done and not always possible.
This is more of a problem in very wet locations such as the OPs (Orlando) or near the ocean with high humidity. The copper wire connection directly to the motor housing could be the source. Especially since it penetrates the paint at that location. It is fairly easy for both of those to get wet at the same time as they are in close proximity to each other and lower on the motor where more water will hit it. It just needs a thin film. Keeping both of these dry would certainly help. I also wonder if they had moved it higher on the motor body, it would have caused less of an issue. The bonding point for my VS pump motor is on the back and top of the motor almost underneath the drive so I would expect that location to be more protected from moisture than a location such as that the Intelliflo uses.
But I agree that the rate of corrosion seems very high pointing to another factor. NEV is another source that could be accelerating the corrosion although AC electrolytic reactions are much less than DC reactions (reason AC is not used for SWGs) but it still might have an impact.
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
I have solar panels, so maybe an additional connection to ground. I'm not sure what's behind it. I did test for an electrical current, and there is none.
I would disconnect the pool’s bonding loop from any part of solar PV system. There’s nothing to be gained from it. The purpose of the bonding wire for the pool is to make sure that everything metallic that is either touching the pool water OR within 5 ft of the pool water is connected together. If something is far away from the pool, then there’s no need to connect it. And, the “grounding” of the electrical system and “bonding” of the pool system are two different things technically speaking. Connecting grounding and bonding is not required nor does it add any benefit.
- Aug 20, 2020
- 7,764
- Pool Size
- 27000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60
Did you check for current or voltage? Where did you measure?I have solar panels, so maybe an additional connection to ground. I'm not sure what's behind it. I did test for an electrical current, and there is none.
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.