Many things are bonded and grounded, so the bonding and grounding are connected and you cannot avoid this.
Electrical issues are incredibly difficult to diagnose, even for experienced electricians.
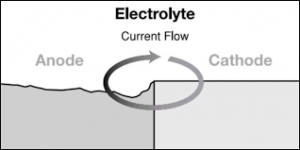
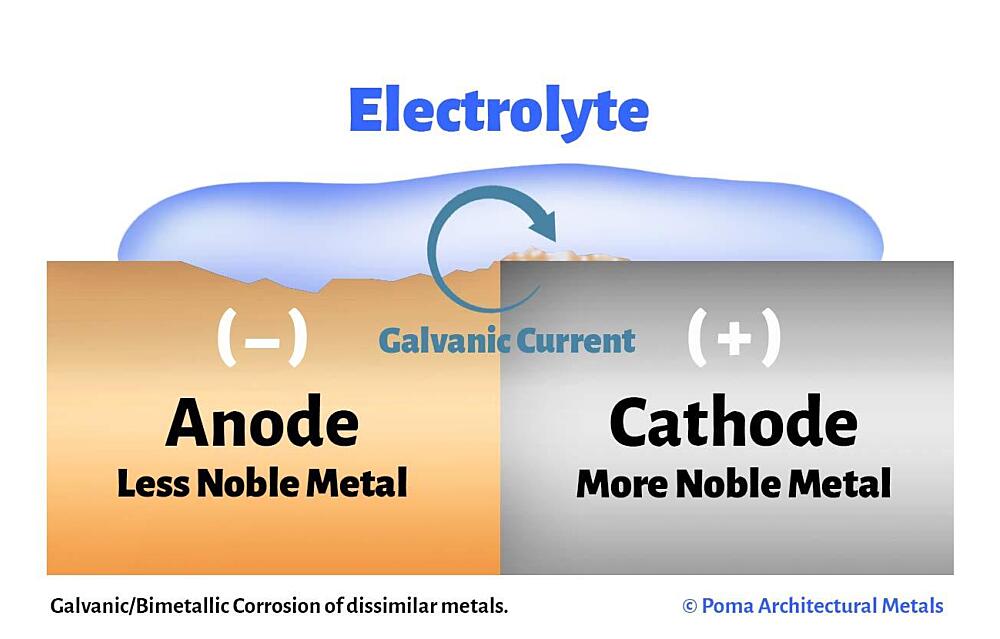
The aluminum is a sacrificial anode to everything else on the bonding grid and the grounding grid.
Oxidation is the loss of electrons.
When an oxidizer, like oxygen, chlorine, hydrogen chloride gas etc. comes in contact with a metal like steel, the iron loses electrons because the oxygen pulls the electrons loose.
This creates iron oxide (aka rust).
If you have a less noble metal like aluminum, zinc or magnesium connected by a wire, then the oxygen pulls the electrons from the less noble metal even though the oxygen is making contact with the steel.
All metal connected to the bonding grid and the grounding system is protected from oxidation by the least noble metal and the least noble metal takes all of the corrosion that would have happened to all more noble metal.
The total amount of corrosion is equal to what it would be if all metal was made from the least noble metal and this is all concentrated on the least noble metal.
The least noble metal acts as a reducing agent to oxidizers like oxygen.
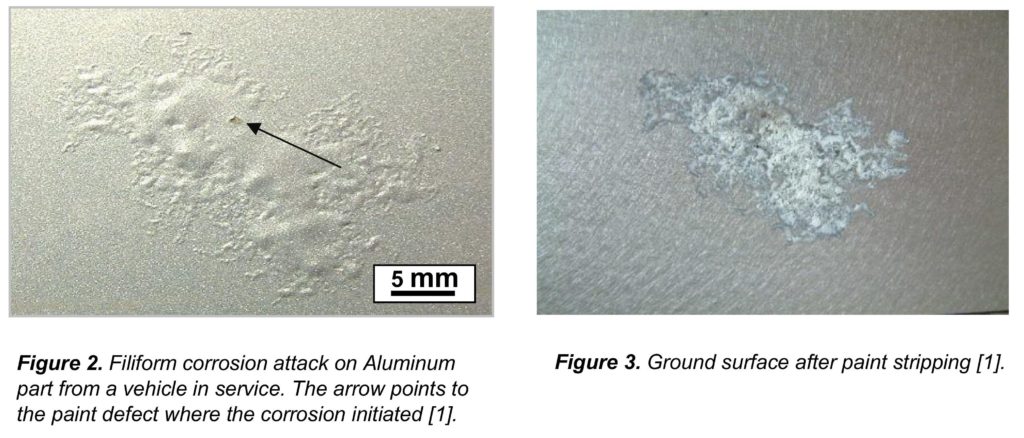
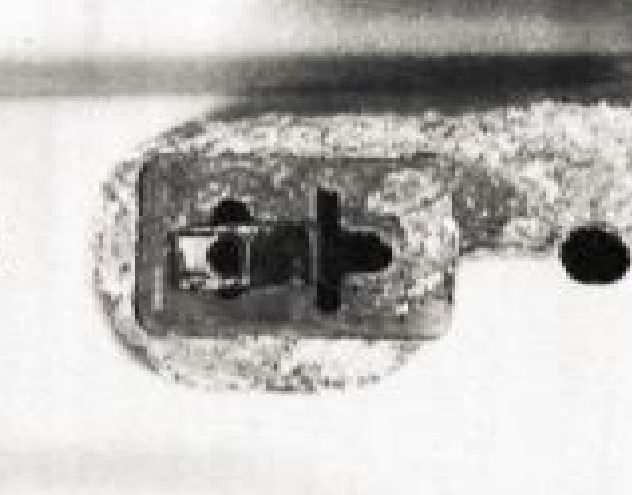
The aluminum casing has a net loss of electrons due to an electrical and/or a chemical problem in the local environment.
Aluminum can form a protective layer of aluminum oxide, which helps prevent surface attack by oxidizers like oxygen because the aluminum in aluminum oxide is already oxidized, however, an electrical problem can reach below the surface and pull out electrons due to the conductivity of the metal.
An electrical problem cannot easily be found and it might be intermittent and only active during certain conditions.
Since everything is bonded and grounded, it’s difficult to isolate any stray current or voltage.


Electrical issues are incredibly difficult to diagnose, even for experienced electricians.
The aluminum is a sacrificial anode to everything else on the bonding grid and the grounding grid.
Oxidation is the loss of electrons.
When an oxidizer, like oxygen, chlorine, hydrogen chloride gas etc. comes in contact with a metal like steel, the iron loses electrons because the oxygen pulls the electrons loose.
This creates iron oxide (aka rust).
If you have a less noble metal like aluminum, zinc or magnesium connected by a wire, then the oxygen pulls the electrons from the less noble metal even though the oxygen is making contact with the steel.
All metal connected to the bonding grid and the grounding system is protected from oxidation by the least noble metal and the least noble metal takes all of the corrosion that would have happened to all more noble metal.
The total amount of corrosion is equal to what it would be if all metal was made from the least noble metal and this is all concentrated on the least noble metal.
The least noble metal acts as a reducing agent to oxidizers like oxygen.
The aluminum casing has a net loss of electrons due to an electrical and/or a chemical problem in the local environment.
Aluminum can form a protective layer of aluminum oxide, which helps prevent surface attack by oxidizers like oxygen because the aluminum in aluminum oxide is already oxidized, however, an electrical problem can reach below the surface and pull out electrons due to the conductivity of the metal.
An electrical problem cannot easily be found and it might be intermittent and only active during certain conditions.
Since everything is bonded and grounded, it’s difficult to isolate any stray current or voltage.