If the R-0007 is off, is very easy to test, as long as you can differentiate the pink in the FC-test from clear. Just take a 10ml pool water sample (after you confirmed that your FC is not zero), and add a few drops of R-0007. Then add a scoop of DPD powder (R-0870). If the R-0007 is OK, then the sample should stay clear. If the sample turns pink, then there is something wrong with the R-0007. You have to do it that way - first R-0007, then DPD powder. Not the other way round - R-0007 won't turn an already pink sample clear.
And the R-0008 you can test as described in my previous post. I once had a bad indicator for the CH-test, and it only created a very faint colour in the water sample.
You seem to have quite a new TF-PRO kit, hard to imagine that you already have bad reagents, but who knows...
Here is a Taylor-video that shows the bleaching effect at high FC (or when not enough or bad R-0007 has been added):
But with an FC of 8ppm that bleaching effect shouldn't happen with two drops of R-0007. Unless you grabbed the wrong bottle when adding R-0007 or something is wrong with the R-0007.
Your colours look similar to those in the Taylor video, but your's start with green.
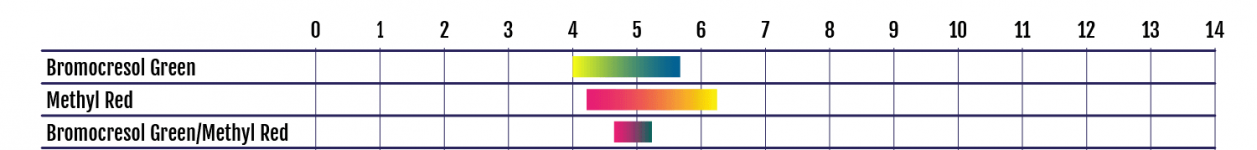
How the colours look like exactly should depend on the exact amount of excess FC (that hasn't been neutralized with R-0007) and to which degree the Methyl Red component of the R-0008 is bleached out and what the exact pH is. Methyl Red is yellow at high pH. The other component in R-0008 is Bromocresol Green which is blue at high pH. Blue and yellow result in green. When the Methyl Red starts to fade with increasing FC, the resulting green becomes fainter and turns eventually into blue. Adding R-0009 to reduce the pH will then turn the Bromocresol Green via greenish/clear to a faint yellow.
Here is a chart showing the colours of the individual indicators in R-0008 and the resulting mixed colours (without chlorine interference) depending on pH:
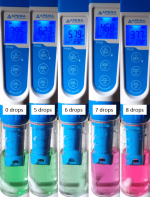
I got curious and ran a quick test. My pool is currently in winter mode where I keep my FC above 10ppm. Just took a sample and ran a TA test without any R-0007. After adding R-0008, the sample turned green (but certainly a more blueish green compared to the vibrant green I got in the second test with R-0007, see below). One drop of R-0009 turned the sample blue. Eventually, the sample turned yellow:



It probably takes an initial pH reduction (by adding R-0009) to make the chlorine bleach the Methyl Red fully out. My TA is currently around 80ppm, and I only used a 10ml sample for this qualitative test, so one drop of R-0009 was enough for that first colour change. With a higher TA and with a regular 25ml sample it might take a few more drops for that initial change from green to blue, and that should not be misinterpreted as the endpoint of the titration. The endpoint in case of high FC (or insufficient amounts of (or bad) R-0007) should be yellow. In the transition from blue to yellow it might even look a bit greenish again before the last drop. I am not aware of any other interferences with the TA test then this effect of un-neutralized chlorine (or bromine).
Here a test from the same water sample with R-0007 added:



After adding adding R-0008 the sample turned into a vibrant green. Second drop didn't change the colour at all. Then the green turned a bit "dirtier" before the sample eventually turned pink.
I tend to think that the chlorine interference is the most likely explanation in your case. Either your FC is higher than you think, you grabbed the wrong bottle when adding R-0007, or there is something wrong with your R-0007. But I would expect the sample to turn yellow eventually when you keep adding R-0009.
Test your R-0007 as described above with DPD powder. If it's OK, then add more R-0007 to your water sample before adding R-0008 and see if you can get the indicator to work in the intended green/pink mode. If that is still not working, then test your R-0008 as described in my previous post (at low pH, you don't even need the distilled water, just add the R-0008 straight to a bit of white vinegar or lemon juice).
Yep, I like my Apera. I'm not colour blind, but have difficulties with the orange shades in the phenol red test. Also means that in my "winter mode" with higher FC I don't have to worry about high FC interfering with the pH-test.
If you have a pH-meter anyway, then you can use it for the TA-test as shown in my previous post, until you understand what's going on with your testing. It can also give you some confidence on how the colour shades are supposed to look like for you. With a meter, you can do the TA-test without R-0007 and R-0008, with R-0009 only. Just make sure that the meter is calibrated at pH 7 and pH 4.