I get it, at least from a purist sorta perspective, so thanks. I'm just not sure how much it matters to me that the relationship is direct or
indirect, since I'm reliably having that experience - and presumably the reason you and others advised me and
@tcat to lower TA and thereby exit that cycle. I'll know for sure after some weeks/months of keeping the TA lower.
Early on I also mentioned my spa spillover/waterfall (also in my signature), about 20 GPM 11 hrs/day, and that aeration is surely contributing to the PH rise. But there is one other data point....
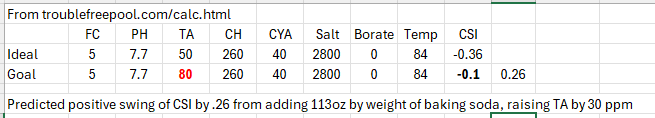
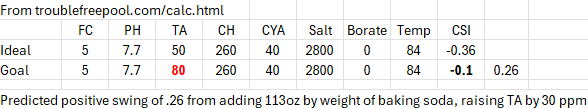
If I plug in some standard "neutral or recommended" values into the original online
poolmath and then add 7 lbs of baking soda to raise the TA 30 ppm, the tool predicts a substantial rise in CSI by +0.26. Granted that's CSI, not PH, influenced by other things (CA & CYA among them), it is still the case that the tool is telling me CSI will rise by by .26 by only adding 30 PPM of TA. The "fix" would always be to check PH, find that it rose (indirectly I guess) then add acid and bring down the PH, thus lowering CSI as well. The cycle, as it were. Perhaps the "app" should at least include the CSI rise as an "estimated effect"? Full values pasted below from that little web tool experiment.
View attachment 622550