You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Also, if you know the details of copper’s behavior in solutions, then copper becomes very very soluble in acidic water. The presence of an oxidizer like chlorine just initiates and feeds into the dissolution of copper metal into water as copper ions. So when you have highly acidic pool water in contact with copper metal, it’s a “perfect storm” of chemistry to cause damage. Most heat exchangers will have a little bit of calcium carbonate scale on the internal surface to protect the metal but that too dissolves quickly at low pH and so there really isn’t a lot of margin of error for the heat exchanger.
It is similar to how Aqua Regia (a mixture of nitric acid and hydrochloric acid), will dissolve gold.
The copper forms a patina of copper oxide and other ionic compounds that protect the elemental copper from oxidation.
At normal pool pH, the ionic copper compounds are insoluble.
At low pH the copper oxides dissolve easily and expose the elemental copper to oxidation by Chlorine, Bromine, Oxygen, Ozone etc.
The copper forms a patina of copper oxide and other ionic compounds that protect the elemental copper from oxidation.
At normal pool pH, the ionic copper compounds are insoluble.
At low pH the copper oxides dissolve easily and expose the elemental copper to oxidation by Chlorine, Bromine, Oxygen, Ozone etc.
Last edited:
A penny will slowly turn brown over time due to the oxidation of copper, which forms a patina.
The patina protects the copper from oxidation.
However, if you put the penny in vinegar, the brown patina will come off in a few minutes to several hours.
The penny will then begin to turn brown again because the elemental copper is exposed to oxygen.
For copper pipe or heat exchangers, it is important to avoid low pH even for a short period of time so that a patina can form and slowly get thicker over time.
There is no doubt that the heat exchanger is corroded, the question is how bad.
Corrosion also creates roughness and pitting, which makes the copper susceptible to flow erosion and to oxidation attack as the rough areas will not maintain a patina as well as smooth areas.
Copper can last for decades with perfect chemistry.
With bad chemistry, copper can be destroyed in hours or days.
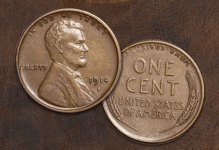

The patina protects the copper from oxidation.
However, if you put the penny in vinegar, the brown patina will come off in a few minutes to several hours.
The penny will then begin to turn brown again because the elemental copper is exposed to oxygen.
For copper pipe or heat exchangers, it is important to avoid low pH even for a short period of time so that a patina can form and slowly get thicker over time.
There is no doubt that the heat exchanger is corroded, the question is how bad.
Corrosion also creates roughness and pitting, which makes the copper susceptible to flow erosion and to oxidation attack as the rough areas will not maintain a patina as well as smooth areas.
Copper can last for decades with perfect chemistry.
With bad chemistry, copper can be destroyed in hours or days.

- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
It's also why copper makes an excellent choice for a buried residential water pipes. Almost all soils, when you bury a pipe deep enough, are neutral to alkaline pH and so copper is extremely stable. Most of the damage that happens to copper water lines is usually from the inside out because the water source chemistry is off or because a brazed fitting fails at the solder joint. The straight sections of pipes themselves will almost never fail. Copper can easily go 50 years underground without ever needing to be replaced.
- Oct 25, 2015
- 5,809
- Pool Size
- 28000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60 Plus
uh-oh... they're at it again. The charts were pretty neat to follow but I bet we'll be seeing mult-colored spirals and tornado diagrams pretty soon!


- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
- Oct 25, 2015
- 5,809
- Pool Size
- 28000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60 Plus
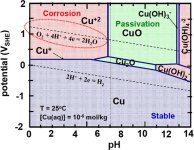
Thanks! I was wondering where the pool was in all this... NOT!Actually ... this one is much more instructive -
View attachment 612754
I added the area where pool water exists ... notice how excruciatingly close pool water is to the region of copper instability. That is why pH is so important for heaters ...

To figure out how negative the TA is, you can do a base demand test.
Fill to 44 ml line and add 3 drops of R-0007 and 9 drops of R-0008.
If the color goes to red immediately, the TA is 0 or lower.
Add R-0006 dropwise and cap and mix until the color changes from red to green.
Multiply the number of drops of base demand by 3.6 to get negative TA.
pH = -log(0.00002 x TA). Use a positive TA number for this calculation.
For example, if it takes 10 drops and your pool is 10,000 gallons, then it would take 3.2 lb sodium carbonate to raise the TA to 0.
3.2 lbs in 10,000 gallons is 36 ppm TA, so each drop is 3.6 ppm and your TA is -36 and the pH is -log(0.00002 x 36) = 3.14.
If you use a 25 ml sample, each drop of base demand is 6.3 ppm.
Based on a 200 CYA, the TA would be estimated at -150 (80 - 230).
If you used a 25 ml sample and did a base demand, the predicted number of drops is 24 drops x 6.3 = -151 ppm TA and a pH of 2.52
If you use a 15.8 ml sample, each drop of base demand is 10 ppm TA.



 www.wolframalpha.com
www.wolframalpha.com
Note: Make sure the log is the base 10 logarithm and not the Natural Logarithm.
pH = -log10(0.00002 x TA).

pH = -log(0.00002x + 0.0000316228)
pH = -log_10(0.00002(TA) + 0.0000316228)
Fill to 44 ml line and add 3 drops of R-0007 and 9 drops of R-0008.
If the color goes to red immediately, the TA is 0 or lower.
Add R-0006 dropwise and cap and mix until the color changes from red to green.
Multiply the number of drops of base demand by 3.6 to get negative TA.
pH = -log(0.00002 x TA). Use a positive TA number for this calculation.
For example, if it takes 10 drops and your pool is 10,000 gallons, then it would take 3.2 lb sodium carbonate to raise the TA to 0.
3.2 lbs in 10,000 gallons is 36 ppm TA, so each drop is 3.6 ppm and your TA is -36 and the pH is -log(0.00002 x 36) = 3.14.
If you use a 25 ml sample, each drop of base demand is 6.3 ppm.
Based on a 200 CYA, the TA would be estimated at -150 (80 - 230).
If you used a 25 ml sample and did a base demand, the predicted number of drops is 24 drops x 6.3 = -151 ppm TA and a pH of 2.52
If you use a 15.8 ml sample, each drop of base demand is 10 ppm TA.



-log(0.00002 x 36) - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
Note: Make sure the log is the base 10 logarithm and not the Natural Logarithm.
pH = -log10(0.00002 x TA).

pH = -log(0.00002x + 0.0000316228)
pH = -log_10(0.00002(TA) + 0.0000316228)
Last edited:
its a mastertemp 250 I believe. I’ll be there on Monday and will suggest an inspection. It’s also a very new heater. It was installed in June.It looks like the customer has an automatic cover.
If that was kept closed, then there would be no chlorine loss due to UV, which increases the chlorine level over an uncovered pool.
Assuming a pH of maybe 2.5 and a FC of 20 ppm, or higher, the copper is definitely compromised.
What is the heater model number?
Some heaters have a sealed can that the heat exchanger sits in and some have a heat exchanger that is more accessible.
Not much you can do about the compromised copper other than watching for leaks.
I need to spend more time understanding your other posts and effective TA vs measured TA.
Thanks for the insight.
What is the best resource to better understand this? Converting measured TA and pH per the test kit, to actual or effective?The main takeaway is that at 0 TA, the pH is 4.5, but it drops fast after the TA is no longer available to buffer the effect of acid.
You lose a full pH unit just by lowering the TA by 15 ppm.
You hit a pH of 3 at -50 ppm.
View attachment 612710
My reference was to when the TA goes below zero.Converting measured TA and pH per the test kit, to actual or effective?
If you do the TA test and the sample goes red immediately, then the TA is zero, or lower.
Since you are in the industry, you will run into this periodically when you get to a new job where the people use trichlor and they do not test the water.
The tests are so that you will be able to see what the actual TA and pH are so that you can figure out a solution.
In most cases, the CYA will be over 100, so a full or partial drain and refill will be necessary if draining can be done safely.
The TA is reported as 80, but I suspect that that is after someone found a negative TA and adjusted it with baking soda.CYA was around 200 I believe. Free chlorine at our last check before draining was 1.5 and TA was 80 and ph was 6.5.
In my opinion, the pH probably went below 3.0, but this is just speculation based on the information provided.
When the CYA is high, you need to figure out the "Adjusted Alkalinity", which is the carbonate alkalinity.
Borate C.F (correction factor) based on pH.
pH.......CF
7.2.....0.051
7.4.......0.0786
7.6......0.1248
7.8......0.1989
Cyanuric Acid correction factor based on pH.
pH........CF
7.0.......0.22
7.1.......0.24
7.2.......0.26
7.3.......0.28
7.4.......0.30
7.5.......0.32
7.6.......0.33
7.7.......0.34
7.8.......0.35
7.9.......0.36
For example, if the pH = 7.6, TA = 90, Borate = 50 and CYA = 70, the adjusted alkalinity is 90 - (70 x 0.33) – (50 x 0.1248) = 60.66.
Borates and Adjusted Alkalinity
Adjusted TA = TA – (CYA X CYA C.F) – (Borate x Borate CF)Borate C.F (correction factor) based on pH.
pH.......CF
7.2.....0.051
7.4.......0.0786
7.6......0.1248
7.8......0.1989
Cyanuric Acid correction factor based on pH.
pH........CF
7.0.......0.22
7.1.......0.24
7.2.......0.26
7.3.......0.28
7.4.......0.30
7.5.......0.32
7.6.......0.33
7.7.......0.34
7.8.......0.35
7.9.......0.36
For example, if the pH = 7.6, TA = 90, Borate = 50 and CYA = 70, the adjusted alkalinity is 90 - (70 x 0.33) – (50 x 0.1248) = 60.66.
To figure out how negative the TA is, you can do a base demand test.
Fill the sample tube to 15.8 ml and add 1 drop of R-0007 and 3 drops of R-0008.
If the color goes to red immediately, the TA is 0 or lower.
Add R-0006 dropwise and swirl to mix until the color changes from red to green.
Multiply the number of drops of base demand by 10 to get negative TA.
pH = -log10(0.00002 x TA).
Use a positive TA number for this calculation.
Note: Make sure the log is the base 10 logarithm and not the Natural Logarithm.
Fill the sample tube to 15.8 ml and add 1 drop of R-0007 and 3 drops of R-0008.
If the color goes to red immediately, the TA is 0 or lower.
Add R-0006 dropwise and swirl to mix until the color changes from red to green.
Multiply the number of drops of base demand by 10 to get negative TA.
pH = -log10(0.00002 x TA).
Use a positive TA number for this calculation.
Note: Make sure the log is the base 10 logarithm and not the Natural Logarithm.
For Carbonate alkalinity, the bicarbonate is in equilibrium with carbon dioxide based on the pH.
Once the pH is below 6.35, carbon dioxide is more than 50% by molar ratio.
X = pH.
Y = %.
The Blue Line is the percentage bicarbonate.
The Yellow Line is the percentage Carbon Dioxide.
HCO3- + H+<--> H2O + CO2aq

 www.wolframalpha.com
www.wolframalpha.com
Once the pH is below 6.35, carbon dioxide is more than 50% by molar ratio.
X = pH.
Y = %.
The Blue Line is the percentage bicarbonate.
The Yellow Line is the percentage Carbon Dioxide.
HCO3- + H+<--> H2O + CO2aq

y = (100/(1+10^(6.35– x))) and y = 100 - (100/(1+10^(6.35– x))), x from 4. to 8. - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
You’re certainly deserving of your expert moniker.For Carbonate alkalinity, the bicarbonate is in equilibrium with carbon dioxide based on the pH.
Once the pH is below 6.35, carbon dioxide is more than 50% by molar ratio.
X = pH.
Y = %.
The Blue Line is the percentage bicarbonate.
The Yellow Line is the percentage Carbon Dioxide.
HCO3- + H+<--> H2O + CO2aq
View attachment 613040
y = (100/(1+10^(6.35– x))) and y = 100 - (100/(1+10^(6.35– x))), x from 4. to 8. - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.www.wolframalpha.com
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.