Purpose of lower TA
- Thread starter xDom
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Sep 23, 2015
- 2,012
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V25
Chlorine is in main-group 7 of the periodic table. That means it is only one electron short of becoming a main-group 8 element with a complete electron shell and achieving the ultimate goal of having the electron structure of a noble gas (Argon in this case). This makes Chlorine a very strong oxidizer, i.e. it likes to steal electrons from other stuff around. In the process, this stuff's "oxidation level" increases, because it's losing negatively charged electrons, i.e. the stuff is getting "oxidized". The Chlorine's oxidation level gets "reduced", it is gaining a negatively charged electron.
This works similarly with Bromine, which is also a main-group 7 element with a similar electron structure as Chlorine, just further "out", the inner shells are all filled. By gaining an electron, it reaches the noble gas-like electron configuration of its neighbour Krypton in the periodic table. But let's stick with Chlorine.
The challenge is now to offer Chlorine in a form that is kind of stable in water, without losing it's oxidizing properties (you don't want the Chlorine to be to content with what it has where it is). That's where HOCl works well. And to a degree also OCl-, the extra electron sits with the Oxyxgen, and the Chlorine in OCl- still has oxidizing powers. But because HOCl is an electrically neutral molecule, it is easier to get through cell membranes and create havoc inside the cell:

I have not seen that before, I hope you don’t mind but I’m keeping a copy of that.
Trying to add to that, think beyond the picture to an army of thousands of those little HOCl molecules that are rushing in to cause havoc. Bound to the HO the Cl is not really content with this de facto relationship, it wants its own electron, is not fussy where it comes from and is happy to dump the HO temporarily arrangement.
There are numerous places to easily steel an electron, one of the first is from the cellular respiration electron transport chain in the mitochondria that is busy making ATP for an energy transport system. This is the equivalent of suffocating a higher order species. Our little electron hungry army will attack anywhere it can, glucose, enzymes, proteins and even the DNA.
- Sep 23, 2015
- 2,012
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V25
You’re messing with my head, I think, or was that thought. Can someone please push me over to the shallow end, I think one of my floaties just fell off…
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
I always admired the creativity of those finding names.
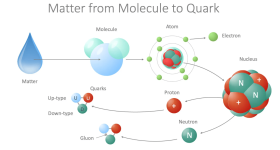
"What should we call those tiny particles that come in threes and make up the nucleons? Hmm, I could earn a few Brownie points in pretending that I have read an unreadable book and call them 'quarks'." Thanks, Murray!
Even better when you know quark as a dairy product, and when understanding what Germans mean by saying "Das ist doch Quark!".
"And what about those particles that create a gooey mess keeping the quarks together? What about 'gluons'?"
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
I'd say neutrinos. When I started uni they still had no mass, when I finished they had a mass and were changing shape. Awesome. Sneaky little buggers. And you need massive pools to find them.
I am creating a system of satellites that can detect neutrinos and they will be able to CAT Scan the earth using solar neutrinos.
This will allow a 3 dimensional model of the earth and all of the internal structures.
The nice thing is that they can beam the data directly to my lab through the earth using neutrino laser beams and my specialized neutrino detectors.
This will allow a 3 dimensional model of the earth and all of the internal structures.
The nice thing is that they can beam the data directly to my lab through the earth using neutrino laser beams and my specialized neutrino detectors.
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
Wouldn't a neutrino laser be awesome.
Imagine Arnie using one. Saving the world with a naser.
Imagine Arnie using one. Saving the world with a naser.
Last edited:
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
I am creating a system of satellites that can detect neutrinos and they will be able to CAT Scan the earth using solar neutrinos.
Imagine Jim Henson had got this idea:
Pools in Space
So if an element had less than 7 electrons in its outer shell, the tendency for it to be an oxidiser is less?Chlorine is in main-group 7 of the periodic table. That means it is only one electron short of becoming a main-group 8 element with a complete electron shell and achieving the ultimate goal of having the electron structure of a noble gas (Argon in this case). This makes Chlorine a very strong oxidizer
If an element has only one electron in its outer shell does it still try to gain an electron or would it try to lose one?
Also the description you gave about the neutral HOCL molecule being suited to breaking down contaminants. You said that because it doesn’t have a charge it has the ability to penetrate the negatively charged outer of the contaminant?
Does this explain why ORP probes are problematic?
Just because the net state of the water is oxidative, doesn’t mean that the water has suitable molecules to penetrate the contaminants? HOCL is what’s needed?
Have I got this right?
Thanks.. ( I remember in high school, back in the late 80’s I had an interest in chemistry. Since getting the pool, Ive reignited this interest , ha! )
Last edited:
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
So if an element had less than 7 electrons in its outer shell, the tendency for it to be an oxidiser is less?
Generally yes, but it's complex. There are sub-shells to be filled, size matters (chlorine is a stronger oxidiser than bromine, fluorine is even stronger, but as the most electronegative element is kind of always already reduced in all the forms you could use it), shape of the unfilled shells matters.
Oxygen has 2 missing electrons, but is still a powerful oxidiser (guess where the name oxidiser comes from), for example in the form of hydrogen peroxide or ozone.
I just have some general chemistry background, I'm sure Matt and could fill pages here on that matter.
If an element has only one electron in its outer shell does it still try to gain an electron or would it try to lose one?
Pretty much. That's how ionic binding works. Chlorine really wants this extra electron, but sodium rather got rid of its extra electron. So, sodium turns into Na+ and chlorine into Cl- and form NaCl. That complete changes the chemical behaviour of the involved elements. We happily pour NaCl over our breakfast eggs, but I wouldn't recommend eating/inhaling metallic sodium or chlorine gas. Do a search on "metallic sodium in water", and you will find some interesting images and videos).
Ionic binding is very different to covalent binding, as for example in Cl2 where both chlorines kind of share an electron, their outer shells form a combined shell ("shells" are nothing else than the electrons' wave functions that you find by solving the Schrödinger equation for that particular Coulomb potential - easy peasy
Also the description you gave about the neutral HOCL molecule being suited to breaking down contaminants. You said that because it doesn’t have a charge it has the ability to penetrate the negatively charged outer of the contaminant?
Pretty much. But again, it's complex.
Does this explain why ORP probes are problematic?
Just because the net state of the water is oxidative, doesn’t mean that the water has suitable molecules to penetrate the contaminants? HOCL is what’s needed?
Have I got this right?
Kind of, yes. ORP just measures the overall ability of the stuff in the water to oxidise stuff, it is not selective to HOCl, which pretty much is what matters. Also very temperature dependant, and just because the potential changes a bit with temperature doesn't mean that there are suddenly more or less available reaction partners. The probe's electrodes also tend to get fouled, for example by CYA, which isn't great for our use case.
Guess what? It's complex.
Well there is a theory that there’s only one electron in the universe and it moves back and forth through time.Generally yes, but it's complex. There are sub-shells to be filled, size matters (chlorine is a stronger oxidiser than bromine, fluorine is even stronger, but as the most electronegative element is kind of always already reduced in all the forms you could use it), shape of the unfilled shells matters.
Oxygen has 2 missing electrons, but is still a powerful oxidiser (guess where the name oxidiser comes from), for example in the form of hydrogen peroxide or ozone.
I just have some general chemistry background, I'm sure Matt and could fill pages here on that matter.
Pretty much. That's how ionic binding works. Chlorine really wants this extra electron, but sodium rather got rid of its extra electron. So, sodium turns into Na+ and chlorine into Cl- and form NaCl. That complete changes the chemical behaviour of the involved elements. We happily pour NaCl over our breakfast eggs, but I wouldn't recommend eating/inhaling metallic sodium or chlorine gas. Do a search on "metallic sodium in water", and you will find some interesting images and videos).
Ionic binding is very different to covalent binding, as for example in Cl2 where both chlorines kind of share an electron, their outer shells form a combined shell ("shells" are nothing else than the electrons' wave functions that you find by solving the Schrödinger equation for that particular Coulomb potential - easy peasy).
Pretty much. But again, it's complex.
Kind of, yes. ORP just measures the overall ability of the stuff in the water to oxidise stuff, it is not selective to HOCl, which pretty much is what matters. Also very temperature dependant, and just because the potential changes a bit with temperature doesn't mean that there are suddenly more or less available reaction partners. The probe's electrodes also tend to get fouled, for example by CYA, which isn't great for our use case.
Guess what? It's complex.
…that’s complex
What If Every Electron Is the Same Electron?
A vaguely plausible thought experiment posits that every electron is one electron moving back and forth through time.
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
Well there is a theory that there’s only one electron in the universe and it moves back and forth through time.
…that’s complex

What If Every Electron Is the Same Electron?
A vaguely plausible thought experiment posits that every electron is one electron moving back and forth through time.www.popularmechanics.com
Thanks for that, hadn't heard about it. Very busy electron, running on a tight schedule.
Here's an adjusted atomic model:

- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
Just looked that up. I did know that the idea of positrons being electrons moving back in time goes back to Wheeler, a concept that Feynman used in his Quantum Electro Dynamic theory. But I didn't know that he brought that up in the context of all electrons actually just being one. Thanks for bringing that up.
- Sep 23, 2015
- 2,012
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V25
Quantum Electro Dynamic theory? - Does that fit in somewhere between a Quark and a Gluon? - Some people have way too much spare time! I’m going to recycle and repurpose that time to take my dog for a walk and if there’s any left over after coffee I might test my pool.
Last edited:
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
QED describes the interaction between electrons and light.
The interaction between quarks and gluons is part of QCD - Quantum Chromodynamics.
All very essential to pool water maintenance...
The interaction between quarks and gluons is part of QCD - Quantum Chromodynamics.
All very essential to pool water maintenance...
I like combining a quark-gluon plasma with a Bose-Einstein Condensate.
Try it, it is amazing.
Try it, it is amazing.
- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
OK, I'll ask the guys in the pool shop about this!All very essential to pool water maintenance...
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.