Why Do I Have Algae With a CC of 0?
CCs are a sign that nitrogen based substances like ammonia or urea have reacted with chlorine and have formed "combined chlorine".
It then takes a certain amount of "free chlorine" to complete the oxidation process which ends basically with nitrogen gas being released.
CCs are common in pools with high bather load, they are usually more of a problem in public pools than in residential pools, especially when following the TFP protocol because there's always enough FC to quickly deal with the little CC that forms.
Maybe after a big pool party, especially with little kids involved that have to much FOMO to go to the bathroom.
Plants, such as algae, create chemicals like glucose from carbon dioxide and water. Using light, the carbon in carbon dioxide oxidizes the oxide into oxygen.
6CO
2 + 6H
2O --> C
6H
12O
6 + 6O
2
Carbon dioxide + water--> glucose + oxygen.
This is how plants make oxygen and store energy in sugars. Most of the bulk of plants is carbon from carbon dioxide. Most of the weight of a tree comes from the air.
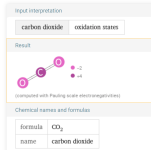
The carbon in carbon dioxide is in the +4 state.
The carbon in glucose is 4 at 0, 1 at -1 and 1 at +1.
The carbon is "reduced", which is the opposite of oxidized.
Reduced just means that the oxidation state is lower or reduced because the atom gained electrons which are negatively charged.
When chlorine oxidizes the carbon in glucose back to a +4 oxidation state, it reverts back to carbon dioxide.
This is a similar process to an animal using oxygen to burn sugars to release energy and exhale carbon dioxide.
So, chlorine reacting with algae is mostly an oxidation reaction and not a combination. Algae is mostly converted back into carbon dioxide and water.
Chlorine can combine with carbon compounds, such as methane (CH
4) by replacing the hydrogen ions.
Carbon in methane is in the -4 oxidation state.
Algae mostly does not create CCs.
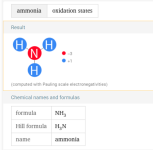
CCs are mostly created by compounds like ammonia where the nitrogen is in the -3 state.
For ammonia, you get combination and oxidation.
So, the CCs eventually go away, especially in sunlight where UV photons knock loose electrons from the nitrogen and make it easier for the chlorine to take them.
Active chlorine is +1, so it bonds with more negatively charged atoms, like nitrogen in the -3 state or carbon in the -4 state.
The carbon in algae has a zero net charge.
So, the reaction of chlorine with algae is mostly oxidation and not combination.
View attachment 517948
______________________________________________________________________________________________________________________
View attachment 517950
______________________________________________________________________________________________________________________
View attachment 517949