So I went on vacation and I assume within a couple of days of returning my flow sensor got chewed through. Chlorine was at .05. No green or visible algae, but I'm sure it's there. I repaired it and set it to super chlorinate until I could pick up some liquid chlorine (sodium hypochlorite 12.5%). I've been putting 3 gallons in my 14,000 gallon above ground and it's going through it in a few hours. The problem I have now is that it is lowering the Ph and has since I installed it this year. I was under the impression it would raise the ph, but I've only had to add ph+ the entire summer. Does this sound right?
To expand on the chlorine, the pool is 92 due to the heatwave. The SWG should be making 2lbs a day on top of the 5 gallons of liquid I put in. Am I just going through a natural process to fix the problem or is something else going on?
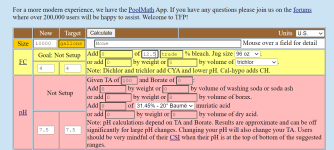
Cyanuric acid less than 30
alkalinity 9
ph 7
To expand on the chlorine, the pool is 92 due to the heatwave. The SWG should be making 2lbs a day on top of the 5 gallons of liquid I put in. Am I just going through a natural process to fix the problem or is something else going on?
Cyanuric acid less than 30
alkalinity 9
ph 7
Last edited: