This might help:
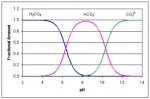
Key:
H
2CO
3=carbonic acid which is CO
2 in water. Dark Blue line
HCO
3-=Bicarbonate. Magenta Line
CO
32-=Carbonate. Green Line
The chart above shows the relative fractions of the various carbonate forms in water versus pH. If you've ever fought pH rise, that middle area with no CO
2 and no carbonate is where your pH wants to settle at 8.3 or so. When the pH moves down toward 6, the carbonic acid fraction starts to increase. So as you move pH down towards 6, some of the carbonate changes to a form that can leave the water as a gas. Aeration speeds up the process.
A key point is that the chart (actually the alkalinity buffering process, the chart is just a visual aid) above works both ways. Changing the fractions changes the pH and changing the pH changes the fractions. If you increase the carbonic acid fraction by injecting CO
2 into the water, the pH will move down. If you increase the carbonate fraction by adding something like sodium carbonate (pHup) then the pH rises.
So when aeration or natural outgassing of CO
2 happens, the pH moves toward the point on the graph that matches the new CO
2 fraction. But alkalinity doesn't change.
This is only the carbonate portion of alkalinity. Alkalinity is also affected by silicates, borates, hydroxide, phosphates and cyanurates. These other ions have their own characteristics and their own pH charts. When you add two buffer systems to your pool, it's possible to put pressure on the pH to stay in a narrower range. This is what happens when you use borates for buffering. The carbonate buffering is much better at keeping the pH from dropping out of our ideal range than it is at keeping it from climbing out of our ideal range, while the borate buffering system helps keep pH from climbing. The combination does a better job of keeping pH close to where we want it than just the carbonate buffer.


