| (122 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | ||
[[Category:Swimming Pool Chemistry]] | [[Category:Swimming Pool Chemistry]] | ||
| − | + | '''To reduce the rate of pH rise, you can reduce your Total Alkalinity until your pH rise is manageable. This article explains the chemistry behind pH rise and reducing Total Alkalinity.''' | |
| − | |||
| − | |||
| − | + | :'''If you want learn how to manage TA and pH, check here [[Managing pH and Total Alkalinity]]''' | |
| − | |||
| − | + | :'''If you want to understand the causes of rising pH and how to slow it, check here [[Managing pH and Total Alkalinity#Causes, and reducing pH rise|Causes, and reducing PH rise]]''' | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | ==Summary== | ||
| + | :'''<big>If your pH rise is rapid and difficult to reasonably manage, reduce your TA to the point your pH rises at a rate that you CAN reasonably manage. Worry about pH (keep it in range) and pH rise (what you can manage), worry less about the absolute TA number (except to keep it at least 50).</big>''' | ||
| + | :1) The higher the Aqueous (dissolved) CO2 levels in the pool, the faster pH will rise. | ||
| + | :2) Lower pH water has higher levels of CO2 | ||
| + | :3) Higher levels of TA allow higher levels of CO2 | ||
| + | :4) When pH is low AND Total Alkalinity is high, the pool has the highest levels of CO2, resulting in the fastest pH rise. | ||
| + | :5) Splashing, water fountains/waterfalls, or slides, high wind, jets pointed up, etc. can cause CO<sub>2</sub> outgassing | ||
| − | |||
==Henry’s Law & Pools== | ==Henry’s Law & Pools== | ||
| − | Henry's law is one of the gas laws formulated by William Henry in 1803 and states: "At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid." | + | :Henry's law is one of the gas laws formulated by William Henry in 1803 and states: "At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid." |
| − | In terms most of us can understand, Henry's law basically states that the amount of a gas dissolved in water wants to be directly proportional to the amount of that same gas in the atmosphere. Our atmosphere has a small percentage of CO2, so CO2 leaves the pool (or outgasses) trying to equalize with the atmosphere. This process is not immediate, it happens over a time. | + | :In terms most of us can understand, Henry's law basically states that the amount of a gas dissolved in water wants to be directly proportional to the amount of that same gas in the atmosphere. Our atmosphere has a small percentage of CO2, so CO2 leaves the pool (or outgasses) trying to equalize with the atmosphere. This process is not immediate, it happens over a time. |
==CO2 and Water== | ==CO2 and Water== | ||
| − | CO2 dissolves in water, and some of it reacts with water molecules to produce a slightly acid solution called carbonic acid. The (aq) indicates water solution. | + | :CO2 dissolves in water, and some of it reacts with water molecules to produce a slightly acid solution called carbonic acid. The (aq) indicates water solution. |
| − | |||
| − | + | ::CO2(aq) + H2O ←→ H2CO3(aq) | |
| − | H2CO3(aq) ←→ H+(aq) + HCO3-(aq) And HCO3-(aq) ←→ H+(aq) + CO3–2(aq) All These are all reversable. | + | :Carbonic acid is weak, so some of it dissociates to produce H+ ions, hence it’s a slightly acidic solution, forming the hydrocarbonate ion, and Bicarbonate, and with release of more H+ ions, Carbonate. |
| + | |||
| + | ::H2CO3(aq) ←→ H+(aq) + HCO3-(aq) And HCO3-(aq) ←→ H+(aq) + CO3–2(aq) | ||
| + | ::::All These are all reversable. | ||
==pH and CO2== | ==pH and CO2== | ||
| − | pH is a measure of how basic/acidic water is. pH is measured by the concentration of Hydrogen (H+) and hydroxyl ions in water. So, the more CO2 in water, the more H+ ions in the water, the more acidic and the lower the pH. | + | :pH is a measure of how basic/acidic water is. pH is measured by the concentration of Hydrogen (H+) and hydroxyl ions in water. So, the more CO2 in water, the more H+ ions in the water, the more acidic and the lower the pH. |
| − | Remember Henry’s law | + | :Remember Henry’s law... Our atmosphere tends to have less CO2 than our pools, so the CO2 in our pools desires to be in equilibrium with the atmosphere, as a result, CO2 outgasses, reducing the H+ ions, increasing pH. |
==pH and TA== | ==pH and TA== | ||
| − | Total alkalinity (TA) is the measure of water's ability to neutralize acids. The role of | + | :Total alkalinity (TA) is the measure of water's ability to neutralize acids. The role of TA is to buffer (slow down) a reduction in pH. When acid is added, carbonate ions can absorb Hydrogen to create bicarbonate ions. And with enough acid, bicarbonate ions absorb another Hydrogen ion and convert into carbonic acid, which is dissolved CO2. The reverse of our equations above. |
| − | Net, net, having alkalis (like Carbonate/Bicarbonate) in the pool allow CO2 to be stored in the pool, | + | :Net, net, having alkalis (like Carbonate/Bicarbonate) in the pool allow CO2 to be stored in the pool. More CO2 in the pool, the more the pool wants to outgas CO2. The more the pool wants to outgas CO2, the faster pH will rise. |
| + | |||
| + | ==Relative Outgas rates based on pH and TA== | ||
| + | |||
| + | As we have established, the rate of outgassing increases with lower pH, higher alkalinity, and aeration of water (splashing, water fountains or slides, high wind, jets pointed up, etc.). The following chart shows the relative outgassing rate as a function of pH and Total Alkalinity. The black line is an arbitrary line, at a relative rate of 15, that we have found is roughly the tolerance limit where many people start complaining about rising pH. Certainly, if you have water features, that line may be lower. | ||
| + | |||
| + | As you can see, lower pH has a larger impact on pH rise than higher TA. | ||
| + | |||
| + | [[File:PH_TA_Relationship_Relative_CO2_Outgassing.png|300 px|thumb]] | ||
==So What?== | ==So What?== | ||
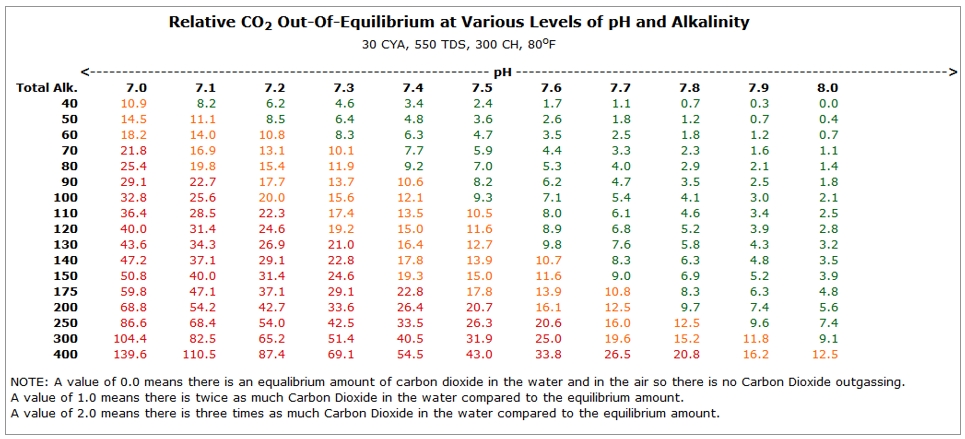
| − | + | :Your pool wants to be in some form of equilibrium, and that is affected by the pH and the TA of the water. If we were to plot HOW FAR OUT OF EQUILIBRIUM the water will be at various levels of TA and pH, it would look like the chart below. | |
| − | The larger the number in the chart, the further the water is out of equilibrium. | + | :The larger the number in the chart, the further the water is out of equilibrium. |
| − | *Perfect equilibrium would be 0 (Zero) on this chart. | + | :*Perfect equilibrium would be 0 (Zero) on this chart. |
| − | *If the number is GREEN (<10), the pool is close to equilibrium. | + | :*If the number is GREEN (<10), the pool is close to equilibrium. |
| − | *If the number is Orange (<20) it is somewhat out of equilibrium. | + | :*If the number is Orange (<20) it is somewhat out of equilibrium. |
| − | *If the number is Red (>20), there is a large pressure on the water to outgas CO2 and you would see a faster rise in pH. | + | :*If the number is Red (>20), there is a large pressure on the water to outgas CO2 and you would see a faster rise in pH. |
| + | |||
| + | :'''<big>If your pH rise is rapid and difficult to reasonably manage, reduce your TA to the point your pH rises at a rate that you CAN reasonably manage. Worry about pH (keep it in range) and pH rise (what you can manage), worry less about the absolute TA number (except to keep it at least 50).</big>''' | ||
[[File:CO2_Out_of_Equilibrium.jpg|Frameles|center|link=https://www.troublefreepool.com/wiki/images/d/d2/CO2_Out_of_Equilibrium.jpg]] | [[File:CO2_Out_of_Equilibrium.jpg|Frameles|center|link=https://www.troublefreepool.com/wiki/images/d/d2/CO2_Out_of_Equilibrium.jpg]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 01:00, 1 May 2025
To reduce the rate of pH rise, you can reduce your Total Alkalinity until your pH rise is manageable. This article explains the chemistry behind pH rise and reducing Total Alkalinity.
- If you want learn how to manage TA and pH, check here Managing pH and Total Alkalinity
- If you want to understand the causes of rising pH and how to slow it, check here Causes, and reducing PH rise
Summary
- If your pH rise is rapid and difficult to reasonably manage, reduce your TA to the point your pH rises at a rate that you CAN reasonably manage. Worry about pH (keep it in range) and pH rise (what you can manage), worry less about the absolute TA number (except to keep it at least 50).
- 1) The higher the Aqueous (dissolved) CO2 levels in the pool, the faster pH will rise.
- 2) Lower pH water has higher levels of CO2
- 3) Higher levels of TA allow higher levels of CO2
- 4) When pH is low AND Total Alkalinity is high, the pool has the highest levels of CO2, resulting in the fastest pH rise.
- 5) Splashing, water fountains/waterfalls, or slides, high wind, jets pointed up, etc. can cause CO2 outgassing
Henry’s Law & Pools
- Henry's law is one of the gas laws formulated by William Henry in 1803 and states: "At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid."
- In terms most of us can understand, Henry's law basically states that the amount of a gas dissolved in water wants to be directly proportional to the amount of that same gas in the atmosphere. Our atmosphere has a small percentage of CO2, so CO2 leaves the pool (or outgasses) trying to equalize with the atmosphere. This process is not immediate, it happens over a time.
CO2 and Water
- CO2 dissolves in water, and some of it reacts with water molecules to produce a slightly acid solution called carbonic acid. The (aq) indicates water solution.
- CO2(aq) + H2O ←→ H2CO3(aq)
- Carbonic acid is weak, so some of it dissociates to produce H+ ions, hence it’s a slightly acidic solution, forming the hydrocarbonate ion, and Bicarbonate, and with release of more H+ ions, Carbonate.
- H2CO3(aq) ←→ H+(aq) + HCO3-(aq) And HCO3-(aq) ←→ H+(aq) + CO3–2(aq)
- All These are all reversable.
- H2CO3(aq) ←→ H+(aq) + HCO3-(aq) And HCO3-(aq) ←→ H+(aq) + CO3–2(aq)
pH and CO2
- pH is a measure of how basic/acidic water is. pH is measured by the concentration of Hydrogen (H+) and hydroxyl ions in water. So, the more CO2 in water, the more H+ ions in the water, the more acidic and the lower the pH.
- Remember Henry’s law... Our atmosphere tends to have less CO2 than our pools, so the CO2 in our pools desires to be in equilibrium with the atmosphere, as a result, CO2 outgasses, reducing the H+ ions, increasing pH.
pH and TA
- Total alkalinity (TA) is the measure of water's ability to neutralize acids. The role of TA is to buffer (slow down) a reduction in pH. When acid is added, carbonate ions can absorb Hydrogen to create bicarbonate ions. And with enough acid, bicarbonate ions absorb another Hydrogen ion and convert into carbonic acid, which is dissolved CO2. The reverse of our equations above.
- Net, net, having alkalis (like Carbonate/Bicarbonate) in the pool allow CO2 to be stored in the pool. More CO2 in the pool, the more the pool wants to outgas CO2. The more the pool wants to outgas CO2, the faster pH will rise.
Relative Outgas rates based on pH and TA
As we have established, the rate of outgassing increases with lower pH, higher alkalinity, and aeration of water (splashing, water fountains or slides, high wind, jets pointed up, etc.). The following chart shows the relative outgassing rate as a function of pH and Total Alkalinity. The black line is an arbitrary line, at a relative rate of 15, that we have found is roughly the tolerance limit where many people start complaining about rising pH. Certainly, if you have water features, that line may be lower.
As you can see, lower pH has a larger impact on pH rise than higher TA.
So What?
- Your pool wants to be in some form of equilibrium, and that is affected by the pH and the TA of the water. If we were to plot HOW FAR OUT OF EQUILIBRIUM the water will be at various levels of TA and pH, it would look like the chart below.
- The larger the number in the chart, the further the water is out of equilibrium.
- Perfect equilibrium would be 0 (Zero) on this chart.
- If the number is GREEN (<10), the pool is close to equilibrium.
- If the number is Orange (<20) it is somewhat out of equilibrium.
- If the number is Red (>20), there is a large pressure on the water to outgas CO2 and you would see a faster rise in pH.
- If your pH rise is rapid and difficult to reasonably manage, reduce your TA to the point your pH rises at a rate that you CAN reasonably manage. Worry about pH (keep it in range) and pH rise (what you can manage), worry less about the absolute TA number (except to keep it at least 50).