| Line 13: | Line 13: | ||
The TA of pool water is raised several ways, primarily through the addition of an Alkaline agent. Depending on what you use, it will have varying effects on pH. This table shows the affect that raising TA by 10 ppm has on pH. | The TA of pool water is raised several ways, primarily through the addition of an Alkaline agent. Depending on what you use, it will have varying effects on pH. This table shows the affect that raising TA by 10 ppm has on pH. | ||
{| class="wikitable" style="text-align: left; color: blue, width: 50%, margin: auto, border-collapse:collapse;" | {| class="wikitable" style="text-align: left; color: blue, width: 50%, margin: auto, border-collapse:collapse;" | ||
| − | |||
| − | |||
! Chemical !! TA increase !! Associated pH increase | ! Chemical !! TA increase !! Associated pH increase | ||
|- | |- | ||
Revision as of 22:51, 31 March 2022
pH TA relationship – The Shallow End
Purpose for looking at the relationship between TA and pH
The primary purposes for looking at the TA and pH relationship are A) knowing how to manage both, because they typically move together, and B) TA and pH, and their relationship, is the number one cause of pool instability (rising pH/Acid demand).
What is the relationship between TA and pH
Maintaining pH within a certain range is important because it can have an effect on plaster, liners, equipment and most importantly on people. TA levels have fewer effects on the pool than pH, however, except in a limited number of situations, TA and pH generally move in tandem. If you increase TA, pH generally increases. If you lower TA, pH generally decreases. This article will explore the relationship between TA and pH, the relationship of TA and pH to pH rise/acid demand, how changing one affects the other, and the impact of the way that you adjust either one.
Adjusting TA will affect pH.
RAISING TA
The TA of pool water is raised several ways, primarily through the addition of an Alkaline agent. Depending on what you use, it will have varying effects on pH. This table shows the affect that raising TA by 10 ppm has on pH.
| Chemical | TA increase | Associated pH increase |
|---|---|---|
| Baking Soda | 10 ppm | .01 ppm increase |
| Soda Ash/Washing Soda | 10 ppm | .35 ppm increase |
| Borax | 10 ppm | .6 ppm increase |
pH TA relationship - The Deep End
Henry’s Law & Pools
Henry's law is one of the gas laws formulated by William Henry in 1803 and states: "At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid."
In terms most of us can understand, Henry's law basically states that the amount of a gas dissolved in water wants to be directly proportional to the amount of that same gas in the atmosphere. Our atmosphere has a small percentage of CO2, so CO2 leaves the pool (or outgasses) trying to equalize with the atmosphere. This process is not immediate, it happens over a time.
CO2 and Water
CO2 dissolves in water, and some of it reacts with water molecules to produce a slightly acid solution called carbonic acid. The (aq) indicates water solution. CO2(aq) + H2O ←→ H2CO3(aq)
Carbonic acid is weak, so some of it dissociates to produce H+ ions, hence it’s a slightly acidic solution, forming the hydrocarbonate ion, and Bicarbonate, and with release of more H+ ions, Carbonate.
H2CO3(aq) ←→ H+(aq) + HCO3-(aq) And HCO3-(aq) ←→ H+(aq) + CO3–2(aq) All These are all reversable.
pH and CO2
pH is a measure of how basic/acidic water is. pH is measured by the concentration of Hydrogen (H+) and hydroxyl ions in water. So, the more CO2 in water, the more H+ ions in the water, the more acidic and the lower the pH.
Remember Henry’s law? Our atmosphere tends to have less CO2 than our pools, so the CO2 in our pools desire to be in equilibrium with the atmosphere, as a result, they outgas, reducing the H+ ions, increasing pH.
pH and TA
Total alkalinity (TA) is the measure of water's ability to neutralize acids. The role of alkalinity (TA to make is simple) is to buffer (slow down) a reduction in pH. When acid is added, carbonate ions can absorb Hydrogen to create bicarbonate ions. And with enough acid, bicarbonate ions absorb another Hydrogen ion and convert into carbonic acid, which is dissolved CO2. The reverse of our equations above.
Net, net, having alkalis (like Carbonate/Bicarbonate) in the pool allow CO2 to be stored in the pool, counterbalancing the pool’s natural desire to outgas, increasing pH.
So What?
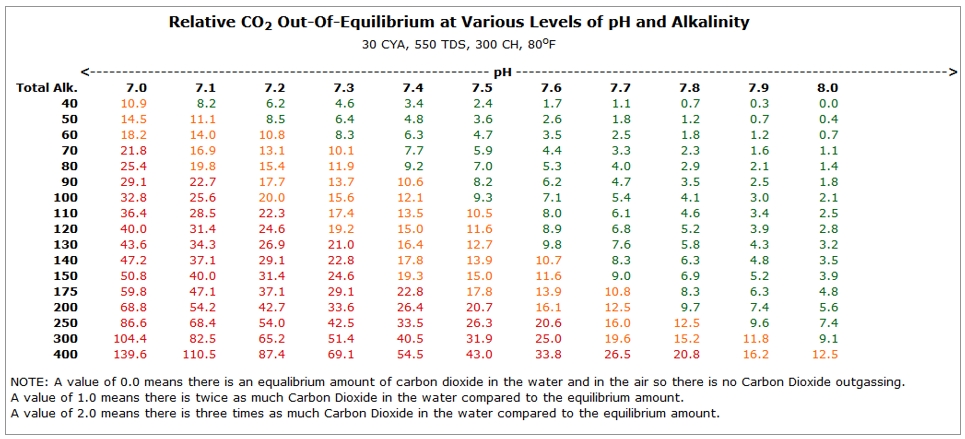
The pool wants to be in equilibrium, and that is affected by the pH and the TA of the water. If we were to plot HOW FAR OUT OF EQUILIBRIUM the water will be at various levels of TA and pH, it would look like the chart below. The larger the number in the chart, the further the water is out of equilibrium.
- Perfect equilibrium would be 0 (Zero) on this chart.
- If the number is GREEN (<10), the pool is close to equilibrium.
- If the number is Orange (<20) it is somewhat out of equilibrium.
- If the number is Red (>20), there is a large pressure on the water to outgas CO2 and you would see a faster rise in pH.
Bringing this all together, you now know: 1) Lower pH water has higher levels of CO2 2) Higher levels of TA allow higher levels of CO2 3) The lower the pH and higher the TA, you will have higher level of CO2 outgassing, resulting faster pH rise.
Understanding the relationship between TA and pH allows you to effectively manage TA and pH levels in your pool.