very important point. Underground waters are usually coming from layers of limestone. It is not a surprise that I have 300 TA and 650 CH in my fillwater. I will seriously consider a filter. Who knows maybe it will be cost efficient in the long run and not dealing with so much acid is a big plus. It is still almost written to the stone in this region to use Sulphuric Acid as the technical rooms are closed locations. Even the government seems to be against it due to HCl vapor build up. No one has complained about health effects of Sulfates so far even though HCl is the chemically natural choice.Isn't it fair to say that a common way for calcium ions to get into the water supply is via calcium carbonate (CaCO3) entering the water and dissolving as Ca2+ and CO32- ions?
The calcium ions will register as CH and the carbonate ions (which will at pool pH actually mostly convert into bicarbonate ions) as TA.
Even though the CH value is independent from bicarbonate, the source for both being in the fill water can be common, as far as I understand. If the water had been flowing through limestone, then it will likely end up being high in both, CH and TA.
Where is CO2 coming from?
- Thread starter fanis.merk
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
That is probably accurate.Isn't it fair to say that a common way for calcium ions to get into the water supply is via calcium carbonate (CaCO3) entering the water and dissolving as Ca2+ and CO32- ions?
The calcium hardness and the total alkalinity can be measured separately and you will likely see a correlation between the two, but you still need to see what each one is and deal with each separately.
The total alkalinity can also come from magnesium carbonate, which will raise the total hardness and the total alkalinity, but it will not raise the calcium hardness.
Calcium carbonate added to water raises the total alkalinity and the calcium hardness by the same amount.Underground waters are usually coming from layers of limestone. It is not a surprise that I have 300 TA and 650 CH in my fillwater.
So, if the calcium hardness is higher than the total alkalinity, then that would suggest that the calcium might be due to something other than calcium carbonate.
Calcium carbonate added to water raises the total alkalinity and the calcium hardness by the same amount.
So, if the calcium hardness is higher than the total alkalinity, then that would suggest that the calcium might be due to something other than calcium carbonate.
Calcium (Ca) and water
Calcium and water: reaction mechanisms, environmental impact and health effects
I think this is an interesting source for this discussion.
Calcium can also come from calcium chloride or calcium sulfate.
Can you test the salinity using a conductivity meter and using a K-1766 salt test kit?
Can you measure the sulfate in the fill water and the pool water?
Can you test the salinity using a conductivity meter and using a K-1766 salt test kit?
Can you measure the sulfate in the fill water and the pool water?
I have Taylor salt test. I measured 1400 salt in fillwater. As far as I know that test shows only Cl concentration. I have nothing to measure sulfates. Don’t have a conductivity meter.Calcium can also come from calcium chloride or calcium sulfate.
Can you test the salinity using a conductivity meter and using a K-1766 salt test kit?
Can you measure the sulfate in the fill water and the pool water?
Based on another discussion we were doing a deep dive in my fillwater. I have 1400ppm Salt in my fillwater based on Taylor salt test. The problem is Taylor test counts Cl. So it is not possible for me to know if it is NaCl or some XCl. Based on CH 650 it is likely to be CaCl. All this time I was adding bags of salt to top up my salt ppm without actually knowing what kind of salt I have in my fillwater. From a SWG perspective does it matter what kind of XCl I have? Will it generate Chlorine if it is not NaCl?
Based on the numbers, the calcium probably comes from a mixture of calcium carbonate and calcium chloride.
Probably. but there is still an excess 1050 ppm that is not accounted for. I mean 300 TA, 650 CH, 1400 XCl. X cannot be all Ca. Probably other minerals are in play as well. Maybe I should go for a professional analysis for contents.Based on the numbers, the calcium probably comes from a mixture of calcium carbonate and calcium chloride.
A water softener can remove the calcium, but it won't do anything for the alkalinity.
You can get equipment that removes carbonates and bicarbonates.
A dealkalizer contains strong base anion exchange resin that exchanges chloride (the Cl ion of the NaCl) for carbonate, bicarbonate and sulfate.
As water passes through the anion resin the carbonate, bicarbonate and sulfate ions are exchanged for chloride ions.
A reverse osmosis machine will remove calcium, carbonate, bicarbonate and most other dissolves solids like chlorides, sulfates, magnesium, sodium etc.
You can get equipment that removes carbonates and bicarbonates.
A dealkalizer contains strong base anion exchange resin that exchanges chloride (the Cl ion of the NaCl) for carbonate, bicarbonate and sulfate.
As water passes through the anion resin the carbonate, bicarbonate and sulfate ions are exchanged for chloride ions.
A reverse osmosis machine will remove calcium, carbonate, bicarbonate and most other dissolves solids like chlorides, sulfates, magnesium, sodium etc.
It is important to remember that each component is reported in its own units.Probably. but there is still an excess 1050 ppm that is not accounted for. I mean 300 TA, 650 CH, 1400 XCl. X cannot be all Ca. Probably other minerals are in play as well.
TA is reported in units of calcium carbonate equivalent.
Calcium is reported in units of calcium carbonate equivalent.
Salt is reported in units of sodium chloride.
If you get a TDS/Salinity meter, that can give you the best idea about the total amount of ions in the water, but it has to be set to either TDS or Salt (NaCl) and those units are not going to be accurate for your water.
A setting of Salt assumes that all TDS is sodium chloride.
A setting of TDS assumes that the TDS is made of 40% sodium sulfate, 40% sodium bicarbonate, 20% sodium chloride.
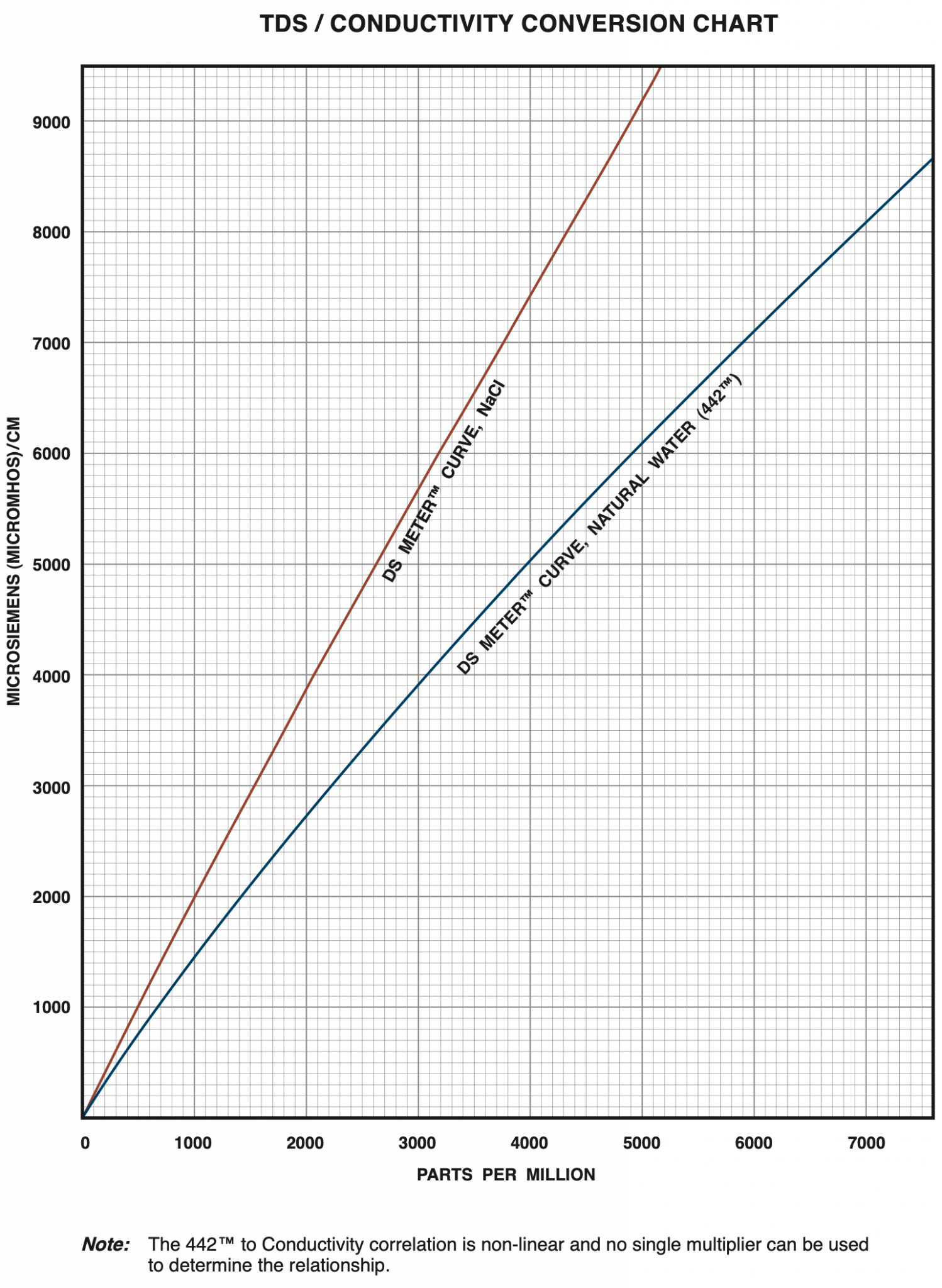
442 Natural Water™ Standard Solution is used in calibrating many Myron L® Instruments. It is the best choice when measuring boiler and cooling water samples, city water supply, lakes, wells, etc. “442” refers to the combination of salts mixed with deionized water to comprise this standard: 40% sodium sulfate, 40% sodium bicarbonate, 20% sodium chloride. A combination of standard salts is necessary since natural water salt type and concentration can vary greatly by location. After much research, the 442 Standard was developed by the Myron L® Company more than 55 years ago. It remains the world’s most accepted standard.

Standard Solutions & Buffers
Click here to download this bulletin as a PDF All Myron L® handheld instruments are factory calibrated with NIST traceable Standard Solutions havingwww.myronl.com
Standard Solutions & Buffers
Ensure specified water testing instrument accuracy. See a list of our Standards Solutions and Buffers (custom solutions available).www.myronl.com
To really analyze the water, you would need to convert each reading to molarity and then balance all of the chemical components.
For example, calcium carbonate is CaCO3, so you would need to know the total number of moles of each ion and then try to find the balance.
Calcium chloride is CaCl2.
So, if you had 4 moles of calcium, 1 mole of carbonate, 1 mole of sulfate and 7 moles of chloride, you could estimate the source is
2CaCl2 + CaCO3 + CaSO4 + 3NaCl --> 4Ca2+ + 3Na+ + CO32- + SO42- + 7Cl-
Last edited:
You can have a variety of ions that increase the total alkalinity without increasing the calcium and you can have compounds that increase the calcium without increasing the total alkalinity.
The K-1766 is specific to chloride, the calcium hardness should be specific to calcium, the TA should be mostly bicarbonate.
If you can test the water using a conductivity meter, this will help estimate the total number of ions in solution.
If you can measure sulfate, that would also help.
Once you get the molarity of each individual ion species, you can try to find a combination of source minerals that will provide the same ratio of dissolved solids.
The K-1766 is specific to chloride, the calcium hardness should be specific to calcium, the TA should be mostly bicarbonate.
If you can test the water using a conductivity meter, this will help estimate the total number of ions in solution.
If you can measure sulfate, that would also help.
Once you get the molarity of each individual ion species, you can try to find a combination of source minerals that will provide the same ratio of dissolved solids.
Last edited:
Somebody moved my SWG question here. Was that you? It is a totally different topic. Can you move it back?You can have a variety of ions that increase the total alkalinity without increasing the calcium and you can have many compounds that increase the calcium without increasing the total alkalinity.
I can't, but maybe a MOD can look at moving it back.Can you move it back?
So would the Taylor test count CaCl2 as NaCl or not?It is important to remember that each component is reported in its own units.
TA is reported in units of calcium carbonate equivalent.
Calcium is reported in units of calcium carbonate equivalent.
Salt is reported in units of sodium chloride.
If you get a TDS/Salinity meter, that can give you the best idea about the total amount of ions in the water, but it has to be set to either TDS or Salt (NaCl) and those units are not going to be accurate for your water.
A setting of Salt assumes that all TDS is sodium chloride.
A setting of TDS assumes that the TDS is made of 40% sodium sulfate, 40% sodium bicarbonate, 20% sodium chloride.
To really analyze the water, you would need to convert each reading to molarity and then balance all of the chemical components.
For example, calcium carbonate is CaCO3, so you would need to know the total number of moles of each ion and then try to find the balance.
Calcium chloride is CaCl2.
So, if you had 3 moles of calcium, 1 mole of carbonate and 7 moles of chloride, you could estimate the source is
2CaCl2 + CaCO3 + 3NaCl --> 3Ca2+ + 3Na+ + CO32- + 7Cl-
I have 1400ppm Salt in my fill water based on Taylor salt test.
1,400 ppm as measured by the K-1766 just measures chloride and reports in units of sodium chloride.I have 300 TA and 650 CH in my fill water.
It does not matter if the chloride came from sodium chloride, calcium chloride, magnesium chloride or potassium chloride.
_________________________________________________
1,400 ppm salt is 1.4 grams per liter of NaCl.
For 80,000 liters, that is 112,000 grams (112 kilograms) of sodium chloride.
Sodium chloride has a Molar mass of 58.44 g/mol
So, you have 1,916.5 moles of chloride in the water.
_________________________________________________
650 Calcium Hardness reported in units of CaCO3 is 0.650 grams per liter.
0.650 x 80,000 = 52,000 grams.
Molar mass: 100.0869 g/mol
That is 519.5 moles of calcium ions.
_________________________________________________
300 TA reported in units of CaCO3 is 0.300 grams per liter.
0.300 x 80,000 = 24,000 grams.
Molar mass: 100.0869 g/mol
That is 239.8 moles of carbonate ions.
_________________________________________________
So, we can say that for every 1 mole of carbonate, you have 2.166 moles of calcium and 8 moles of chloride in the water.
So, we can estimate the TDS (Total Dissolved Solids) in the fill water to be 1 part calcium carbonate, 1 part calcium chloride and 6 parts sodium chloride.
CaCO3 + CaCl2 + 6NaCl --> 2Ca2+ + 6Na+ + CO32- + 8Cl-
Wow. You must be a chem genius. A very good approximation to my test results assuming that NaCl is the missing compound. Thanks for your help. One last thing: is the type of compound providing the Cl- affect the SWG or will I get my Chlorine anyhow?1,400 ppm as measured by the K-1766 just measures chloride and reports in units of sodium chloride.
It does not matter if the chloride came from sodium chloride, calcium chloride, magnesium chloride or potassium chloride.
_________________________________________________
1,400 ppm salt is 1.4 grams per liter of NaCl.
For 80,000 liters, that is 112,000 grams (112 kilograms) of sodium chloride.
Sodium chloride has a Molar mass of 58.44 g/mol
So, you have 1,916.5 moles of chloride in the water.
_________________________________________________
650 Calcium Hardness reported in units of CaCO3 is 0.650 grams per liter.
0.650 x 80,000 = 52,000 grams.
Molar mass: 100.0869 g/mol
That is 519.5 moles of calcium ions.
_________________________________________________
300 TA reported in units of CaCO3 is 0.300 grams per liter.
0.300 x 80,000 = 24,000 grams.
Molar mass: 100.0869 g/mol
That is 239.8 moles of carbonate ions.
_________________________________________________
So, we can say that for every 1 mole of carbonate, you have 2.166 moles of calcium and 8 moles of chloride in the water.
So, we can estimate the TDS (Total Dissolved Solids) in the fill water to be 1 part calcium carbonate, 1 part calcium chloride and 6 parts sodium chloride.
CaCO3 + CaCl2 + 6NaCl --> 2Ca2+ + 6Na+ + CO32- + 8Cl-
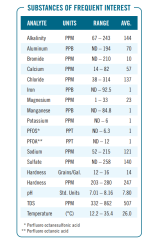
Many places will Produce an annual Water Quality Report that shows all of the different substances that are in the water.
If you have a full report of all substances and dissolved solids, you can convert all to molarity and then try to find a balanced equation that satisfies all of the reported levels.
Below is a sample of a Water Quality Report that reports the levels of the major constituents of the dissolved solids.
You can go through and convert the readings into moles and then figure out the ratio of the individual compounds that would produce the same result.
You would have compounds like calcium chloride, calcium sulfate, calcium carbonate, sodium chloride, magnesium chloride etc.
The more test results that you have for specific compounds, the more accurate the analysis can be.
For example, if you measured for sulfates, you could estimate the amount of calcium sulfate.
If you measure Total Hardness, you can subtract the Calcium Hardness to get the Magnesium Hardness.
If you have a full report of all substances and dissolved solids, you can convert all to molarity and then try to find a balanced equation that satisfies all of the reported levels.
Below is a sample of a Water Quality Report that reports the levels of the major constituents of the dissolved solids.
You can go through and convert the readings into moles and then figure out the ratio of the individual compounds that would produce the same result.
You would have compounds like calcium chloride, calcium sulfate, calcium carbonate, sodium chloride, magnesium chloride etc.
The more test results that you have for specific compounds, the more accurate the analysis can be.
For example, if you measured for sulfates, you could estimate the amount of calcium sulfate.
If you measure Total Hardness, you can subtract the Calcium Hardness to get the Magnesium Hardness.

Last edited:
The SWG only cares about the chloride and it does not matter what compound provided the chloride.One last thing: is the type of compound providing the Cl- affect the SWG or will I get my Chlorine anyhow?
If you used a conductivity meter on the water and got 2,000 ppm in NaCl setting, then you can convert that to total moles, but 2,000 ppm in NaCl is about 2,900 ppm in the “442” standard “TDS” Setting.
So, you can get an estimate of the total number of moles in solution and then try to balance that against the total number of moles of all constituent ions.
The only significant missing measurements are probably magnesium and sulfate.
In any case, the estimate of 1 part calcium carbonate, 1 part calcium chloride and 6 parts sodium chloride for the TDS (Total Dissolved Solids) in the fill water is probably pretty close.
2,000 NaCl is 2 grams per liter.
For 80,000 liters, that is 160,000 grams (160 kilograms) of sodium chloride.
Sodium chloride has a Molar mass of 58.44 g/mol
So, you have 2,737.85 moles of sodium and 2,737.85 moles of chloride in the water for a total of 5,475.7 moles of dissolved ions.
You have 1,916.5 moles of chloride, 519.5 moles of calcium ions, 239.8 moles of carbonate ions,
Based on the balanced equation, the total number of moles of sodium should be 1,439.
So, the total moles are 1,439 + 1,916.5 + 519.5 + 239.8 = 4,114.8
5,475.7 - 4,114.8 = 1,360.9 moles missing.
However, if we count the calcium and carbonate twice since they have double charge, the equation is
So, the total moles are 1,439 + 1,916.5 + (519.5 X 2) + (239.8 X 2) = 4874.1
5,475.7 - 4,874.1 = 601.6 moles.
So, this would suggest a total number of about 600 moles of unaccounted for dissolved solids like maybe sulfate, magnesium etc.
However, this is only a very rough guide and measuring the sulfate and magnesium would give you a better result.

 www.myronl.com
www.myronl.com

So, you can get an estimate of the total number of moles in solution and then try to balance that against the total number of moles of all constituent ions.
The only significant missing measurements are probably magnesium and sulfate.
In any case, the estimate of 1 part calcium carbonate, 1 part calcium chloride and 6 parts sodium chloride for the TDS (Total Dissolved Solids) in the fill water is probably pretty close.
2,000 NaCl is 2 grams per liter.
For 80,000 liters, that is 160,000 grams (160 kilograms) of sodium chloride.
Sodium chloride has a Molar mass of 58.44 g/mol
So, you have 2,737.85 moles of sodium and 2,737.85 moles of chloride in the water for a total of 5,475.7 moles of dissolved ions.
You have 1,916.5 moles of chloride, 519.5 moles of calcium ions, 239.8 moles of carbonate ions,
Based on the balanced equation, the total number of moles of sodium should be 1,439.
So, the total moles are 1,439 + 1,916.5 + 519.5 + 239.8 = 4,114.8
5,475.7 - 4,114.8 = 1,360.9 moles missing.
However, if we count the calcium and carbonate twice since they have double charge, the equation is
So, the total moles are 1,439 + 1,916.5 + (519.5 X 2) + (239.8 X 2) = 4874.1
5,475.7 - 4,874.1 = 601.6 moles.
So, this would suggest a total number of about 600 moles of unaccounted for dissolved solids like maybe sulfate, magnesium etc.
However, this is only a very rough guide and measuring the sulfate and magnesium would give you a better result.


Standard Solutions & Buffers
Click here to download this bulletin as a PDF All Myron L® handheld instruments are factory calibrated with NIST traceable Standard Solutions having

Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

