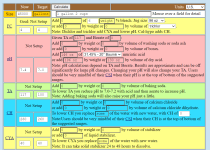
Hello, I have a 45,000 gallon sand filter pool; the pool is concrete. Soda Ash spilled into the pool on accident. Needless to say the pool is cloudy. Around 315oz of soda ash was added directly to the pool. I used 150oz muriatic acid. Reading before soda ash was ->
FC: 5 CC: 5 pH: 7.4 TA: 80 CH: 250
After soda ash and muriatic acid my
TA: 110 CH: 275
What should I do to fix this as quick as possible? I appreciate the help thank you!
FC: 5 CC: 5 pH: 7.4 TA: 80 CH: 250
After soda ash and muriatic acid my
TA: 110 CH: 275
What should I do to fix this as quick as possible? I appreciate the help thank you!