Going through the forums I see a lot of Questions regarding cool owners that have very high calcium levels and people who struggle to get the calcium in line. Theoretically chemistry wise, adding soda ash to the pool should raise the pH locally enough which makes the pool cloudy and traverse in a wave from the point of addition to the rest of the pool. This cloudiness should be the reaction of calcium with a carbonate ion from the soda ash forming calcium carbonate which is insoluble and would precipitate out of the pool to the bottom where can be vacuumed up or through the skimmer filter and flushed out. This should lower the calcium content. After calcium carbonate is formed and the pH can be adjusted back down using muriatic acid and if total alkalinity is too high can be adjusted in the same manner dump chemical in and this should lower the calcium content. After calcium carbonate is formed and removed, the pH can be adjusted back down using muriatic acid and if total alkalinity is too high can be adjusted in the same manner. Chemistry why’s it should leave 50 to 80 ppm of calcium if enough soda ash is added.
My Background: Chemical Engineer
Some References:
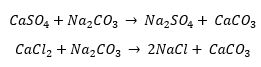
 sodimate-inc.com
sodimate-inc.com
See attached pdf.
Does anyone experienced see any issues with this method?
My Background: Chemical Engineer
Some References:
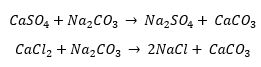
Water Softening - Sodium Carbonate - Soda Ash | Sodimate
Sodimate feed systems are widely used with sodium carbonate to remove hardness from water caused by calcium and magnesium minerals.
 sodimate-inc.com
sodimate-inc.com
See attached pdf.
Does anyone experienced see any issues with this method?

