Hi friends,
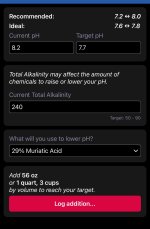
I’ve got my trusty TF-Pro test kit, ever since, my chlorine and CYA levels have been perfect. Tested my total alkalinity tonight and it came back at 240… whoa. So, I tested my pH to verify but realized it’s not a very accurate test and is subject to interpretation. It also maxes out at 8.2. Two questions:
1) within this test kit is there a more accurate test for pH?
2) what on earth do I do about a TA at 240?
I’ve got my trusty TF-Pro test kit, ever since, my chlorine and CYA levels have been perfect. Tested my total alkalinity tonight and it came back at 240… whoa. So, I tested my pH to verify but realized it’s not a very accurate test and is subject to interpretation. It also maxes out at 8.2. Two questions:
1) within this test kit is there a more accurate test for pH?
2) what on earth do I do about a TA at 240?