In 2021 Richard Falk @chem geek published a chart illustrating pH ceilings for different levels of alkalinity. A copy of the chart can be found here: CO2 and pH: Understanding Henry's Law
Would there be any harm in targeting the following metrics to lower the pH ceiling and and reduce the demand for acid?
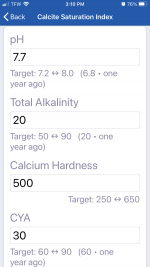
pH 7.7
CH 500
TA 20
CYA 10
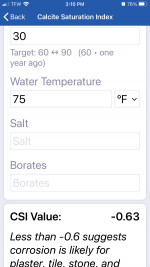
CSI -0.39
Would there be any harm in targeting the following metrics to lower the pH ceiling and and reduce the demand for acid?
pH 7.7
CH 500
TA 20
CYA 10
CSI -0.39