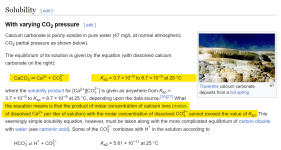
Calcium carbonate solubility is based on the K
sp, which is the Molarity of the calcium ions multiplied by the Molarity of the Carbonate ions.
The K
sp for Calcium carbonate is not a single number and it varies based on different conditions and even by which source you look at.
The calcium ion molarity can be calculated from the calcium hardness reading.
The calcium hardness reading is given in ppm units of calcium carbonate equivalents.
You can convert this to calcium carbonate molarity, which is equal to the calcium ion molarity.
For example, if the calcium hardness is 250 ppm, that is 250 milligrams per liter or 0.00249783M (Moles per liter).
CaCO3 = 100.0869 g/mol.
0.00249783 X (Carbonate ion Molarity) = 3.3 x 10
-9.
Carbonate ion Molarity = 0.00000132114 Moles per liter.
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.

www.wolframalpha.com
The carbonate ion molarity can be calculated from the carbonate alkalinity and the pH.
The carbonate alkalinity reading is also given in ppm units of calcium carbonate equivalents.
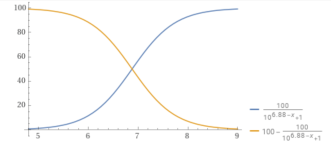
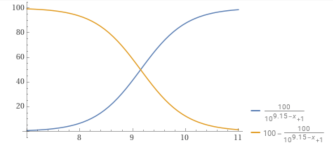
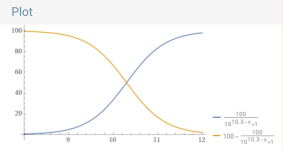
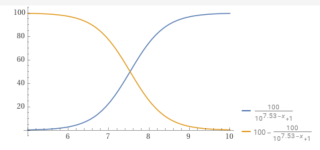
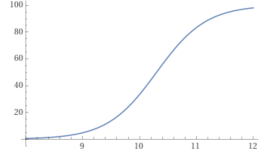
You can plot the percentage of carbonate vs. the pH.
y = (100/(1+10^(10.3– x))).
For example, at a pH of 8.7, the percentage of carbonate is 2.45%.
pH.........% carbonate.
8.0...............0.49868
8.3...............0.99
8.7...............2.45
9.0...............4.77
9.5...............13.68
10...............33.386
10.3...............50
11...............83.36
11.5...............94.0649
12..................98.04
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.

www.wolframalpha.com
So, as you can see, the pH determines how much of the bicarbonate becomes carbonate.
The carbonate concentration multiplied by the calcium ion concentration is equal to the K
sp for Calcium carbonate when the water is fully saturated.
When the carbonate concentration multiplied by the calcium ion concentration is greater than the K
sp for Calcium carbonate, the calcium carbonate will begin to come out of solution as scale and precipitate.
When the carbonate concentration multiplied by the calcium ion concentration is less than the K
sp for Calcium carbonate, the calcium carbonate should remain in solution.