We had Intellichem Controllers installed on 2 pools this past summer. They are set up to dose by Time... one doses liquid chlorine (outdoor pool) and one doses bromine (indoor (pool). They share a common acid tank. I'm interested to know what are ideal or recommended Dosing and Mixing Times. Right now they are set to Dose for 3 minutes and Mix for 5 minutes. Are those good settings or should I consider changing? Thank you.
Intellichem Controller Settings
- Thread starter JPMorgan
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Jul 21, 2013
- 65,181
- Pool Size
- 35000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
You just need to test the levels while the IntelliChem is running and adjust it to get the levels you desire.
How do you dose bromine?one doses bromine (indoor (pool).
There is a solenoid valve that activates the bromine feeder when the controller calls for sanitizer.How do you dose bromine?
It is not a good idea to close a valve on a tab feeder because the tabs are soaking in water and dissolving.There is a solenoid valve that activates the bromine feeder when the controller calls for sanitizer.
This creates a very corrosive soup of low pH and high chlorine/bromine in the feeder.
Hmmmm.... the indoor pool has been operating for the past 8 years with this type of system... bromine feeder/ solenoid valve/ controller. What kind of problems might occur? I am not aware of any issues that have come up in the past 3 years since I have been here... but maybe there are issues that I am unaware of. I do know that the bromine feeder is never filled all the way, but only about half way. Does that mitigate any potential problems? Is there another way to operate a bromine feeder with a controller that does not use a solenoid valve?It is not a good idea to close a valve on a tab feeder because the tabs are soaking in water and dissolving.
This creates a very corrosive soup of low pH and high chlorine/bromine in the feeder.
I recently came across this in my continuing research on maintaining a bromine pool:
“Sodium bromide levels can increase to undesirable levels.... This requires regular dilution, and monitoring (lab tests to confirm that sodium bromide levels stay below 20 ppm). The pool should be drained completely if sodium bromide levels exceed 50 ppm.”
So.... I have recently implemented a regular dilution procedure, i.e., backwashing 500 gallons per week (2.5% of total pool volume) from the pool, but is it really necessary to test for sodium bromide? All I see online for testing sodium bromide are test strips and I know TFP frowns on the use of test strips.
“Sodium bromide levels can increase to undesirable levels.... This requires regular dilution, and monitoring (lab tests to confirm that sodium bromide levels stay below 20 ppm). The pool should be drained completely if sodium bromide levels exceed 50 ppm.”
So.... I have recently implemented a regular dilution procedure, i.e., backwashing 500 gallons per week (2.5% of total pool volume) from the pool, but is it really necessary to test for sodium bromide? All I see online for testing sodium bromide are test strips and I know TFP frowns on the use of test strips.
- Jul 21, 2013
- 65,181
- Pool Size
- 35000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
All I see online for testing sodium bromide are test strips and I know TFP frowns on the use of test strips.
They are better than nothing and if test strips say your bromide levels are high they probably are.
I am not sure why the bromide level would be a problem.
Ask the person making the claim to explain why it is a problem.
https://www.alisonosinski.com/?p=29...ion, and,bromoform forms in brominated pools.
I am not sure that a sodium bromide test kit will identify only bromide and not include chloride, so you need to check with the test kit maker to see if the test kit can identify only the bromide and not other ions like chloride.
Ask the person making the claim to explain why it is a problem.
https://www.alisonosinski.com/?p=29...ion, and,bromoform forms in brominated pools.
I am not sure that a sodium bromide test kit will identify only bromide and not include chloride, so you need to check with the test kit maker to see if the test kit can identify only the bromide and not other ions like chloride.
The bromide level might be related to the formation of bromate.
Bromate is more likely with UV or Ozone.
1 lb of BCDMH (Bromine Tabs) is equivalent to adding 0.4261 lbs of sodium bromide.
50 lb of BCDMH (Bromine Tabs) is equivalent to adding 21.3 lbs of sodium bromide.
21.3 lbs of sodium bromide is 127 ppm sodium bromide in 20,000 gallons.




When ultraviolet (UV) light is used in a water treatment process that contains bromide ions, it can contribute to the formation of bromate, a potentially carcinogenic disinfection byproduct, particularly when combined with other oxidants like chlorine, as the UV radiation creates reactive radicals that can oxidize bromide into bromate; essentially, UV light can facilitate the process of turning bromide into bromate, especially in a UV/chlorine disinfection system.
Key points about UV and bromate formation:
Mechanism:
UV light breaks down water molecules, generating highly reactive hydroxyl radicals (OH•) which can then react with bromide ions (Br-) to form bromate (BrO3-).
Combined processes:
Bromate formation is especially significant when UV is used alongside chlorine, as the chlorine can further react with bromide to create additional reactive species that contribute to bromate production.
Factors influencing bromate formation:
Bromide concentration: Higher bromide levels in the water lead to increased bromate formation.
pH level: Higher pH values generally promote bromate formation.
UV intensity: Increased UV radiation can result in higher bromate production.

 pubmed.ncbi.nlm.nih.gov
pubmed.ncbi.nlm.nih.gov
Bromate is more likely with UV or Ozone.
1 lb of BCDMH (Bromine Tabs) is equivalent to adding 0.4261 lbs of sodium bromide.
50 lb of BCDMH (Bromine Tabs) is equivalent to adding 21.3 lbs of sodium bromide.
21.3 lbs of sodium bromide is 127 ppm sodium bromide in 20,000 gallons.




When ultraviolet (UV) light is used in a water treatment process that contains bromide ions, it can contribute to the formation of bromate, a potentially carcinogenic disinfection byproduct, particularly when combined with other oxidants like chlorine, as the UV radiation creates reactive radicals that can oxidize bromide into bromate; essentially, UV light can facilitate the process of turning bromide into bromate, especially in a UV/chlorine disinfection system.
Key points about UV and bromate formation:
Mechanism:
UV light breaks down water molecules, generating highly reactive hydroxyl radicals (OH•) which can then react with bromide ions (Br-) to form bromate (BrO3-).
Combined processes:
Bromate formation is especially significant when UV is used alongside chlorine, as the chlorine can further react with bromide to create additional reactive species that contribute to bromate production.
Factors influencing bromate formation:
Bromide concentration: Higher bromide levels in the water lead to increased bromate formation.
pH level: Higher pH values generally promote bromate formation.
UV intensity: Increased UV radiation can result in higher bromate production.

Bromate formation from the oxidation of bromide in the UV/chlorine process with low pressure and medium pressure UV lamps - PubMed
When a bromide-containing water is treated by the ultraviolet (UV)/chlorine process, hydroxyl radicals (HO) and halogen radicals such as Cl or Br are formed due to the UV photolysis of free halogens. These reactive species may induce the formation of bromate, which is a probable human...
Attachments
Has that reduced the formation of combined bromine products?I have recently implemented a regular dilution procedure, i.e., backwashing 500 gallons per week (2.5% of total pool volume) from the pool
Your link is where I found the information about keeping a close eye on sodium bromide levels and draining the pool if it reaches 50 ppm.I am not sure why the bromide level would be a problem.
Ask the person making the claim to explain why it is a problem.
https://www.alisonosinski.com/?p=29...ion, and,bromoform forms in brominated pools.
I am not sure that a sodium bromide test kit will identify only bromide and not include chloride, so you need to check with the test kit maker to see if the test kit can identify only the bromide and not other ions like chloride.
Tabs add bromide and DMH.
Since you need to limit the DMH, it will also limit the bromide.
I would not use tabs.
If you use tabs, 20 lbs of tabs will add 50 ppm of sodium bromide.
So, the maximum use of tabs to keep the DMH and bromide low, is 20 lbs from a fresh fill.
Since you need to limit the DMH, it will also limit the bromide.
I would not use tabs.
If you use tabs, 20 lbs of tabs will add 50 ppm of sodium bromide.
So, the maximum use of tabs to keep the DMH and bromide low, is 20 lbs from a fresh fill.
Yes.... I think so. When I test for bromine, I'm getting consistent readings of 5-6 ppm. When I test for combined bromine the water sample clears with 1 -2 drops of R-003. That would be less than 1 ppm, right?Has that reduced the formation of combined bromine products?
Keep diluting on a regular basis to lower the DMH and bromide levels.
Has the algae problem continued or is it resolved?
Has the algae problem continued or is it resolved?
I'm guessing we go through 20 lbs of tabs in about 4 months. (I'm going to monitor this more closely when I start a new 50 lb bucket.) If I'm diluting 2.5% of the water weekly (500 gallons of the 20,000 gallon pool volume), how does this change things? That's 20,000 gallons of fresh water about every 10 months! In a previous post I thought you indicated that with proper water dilution, the water may never need to be drained.Tabs add bromide and DMH.
Since you need to limit the DMH, it will also limit the bromide.
I would not use tabs.
If you use tabs, 20 lbs of tabs will add 50 ppm of sodium bromide.
So, the maximum use of tabs to keep the DMH and bromide low, is 20 lbs from a fresh fill.
It depends on what you are adding.In a previous post I thought you indicated that with proper water dilution, the water may never need to be drained.
It gets complicated when you already have high levels of DMH and you are continuously adding more and diluting at the same time.
Dilution removes DMH, but the amount removed depends on the concentration and how much water is removed.
If you do all of the calculations and dilute the correct amount to remove the amount added, then you can go indefinitely without ever needing a complete water replacement.
For example, if you remove 1,000 gallons of water containing 200 ppm of DMH, you have removed 1.68 lb of DMH, which is 10 ppm.
So, now you have 190 ppm of DMH.
If you add 10 ppm of DMH before your next dilution, then you are back to 200 ppm.
If you remove 1,000 gallons of water containing 50 ppm of DMH, you have removed 0.42 lb of DMH, which is 2.5 ppm.
So, now you have 47.5 ppm DMH.
If you add 10 ppm of DMH before your next dilution, then you are at 57.5 ppm.
If you remove 4,000 gallons of water containing 50 ppm of DMH, you have removed 1.68 lb of DMH, which is 10 ppm.
So, the target equilibrium is the basis for how much dilution is required.
So, you need to know your starting DMH levels, the amount you drain and the amount of DMH you add back to the water to keep track of the level since there is no test for the level of DMH.
This is all complicated and why I would just avoid tabs altogether.
So, now you have 190 ppm of DMH.
If you add 10 ppm of DMH before your next dilution, then you are back to 200 ppm.
If you remove 1,000 gallons of water containing 50 ppm of DMH, you have removed 0.42 lb of DMH, which is 2.5 ppm.
So, now you have 47.5 ppm DMH.
If you add 10 ppm of DMH before your next dilution, then you are at 57.5 ppm.
If you remove 4,000 gallons of water containing 50 ppm of DMH, you have removed 1.68 lb of DMH, which is 10 ppm.
So, the target equilibrium is the basis for how much dilution is required.
So, you need to know your starting DMH levels, the amount you drain and the amount of DMH you add back to the water to keep track of the level since there is no test for the level of DMH.
This is all complicated and why I would just avoid tabs altogether.
C5H8N2O2 (DMH).
CAS Number: 77-71-4
Molecular Weight: 128.13
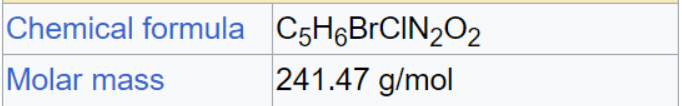
128.13/241.47 = 53.06% DMH.
If you use tabs, 20 lbs of tabs will add 10.612 lb of DMH, which is 63 ppm DMH and 50 ppm of sodium bromide.
To remove 10 ppm of DMH, you need to remove 3,175 gallons and add fresh water.
Molar mass: 285.923 g·mol−1
Formula: C5H6Br2N2O2
Symbol: Br. Atomic mass: 79.904 grams per mole.
79.904 x 4 = 319.616 (2Br2).
319.616/241.47 = 132.36% "Available Bromine" as Br2.
53.06% + 132.36% = 185.42%.
C5H6BrClN2O2 + 2H+ + 3Br- --> C5H8N2O2 + Cl- + 2Br2.
One mole of BCDMH provides 1 mole of DMH, 1 mole of bromide (after reduction) and 1 mole of chloride.
It also provides 2 moles of bromine (Br2) based on how bromine is reported.
2 moles of Br2 = 319.616 grams, which is why the percentage of available bromine is higher than 100% of the mass of BCDMH.
319.616/285.923 = 111%.
1 mole of DBDMH or 1 mole of BCDMH provides 2 moles of Br2.
20 lbs of tabs will add 26.47 lb of bromine, which is 158 ppm bromine.
CAS Number: 77-71-4
Molecular Weight: 128.13
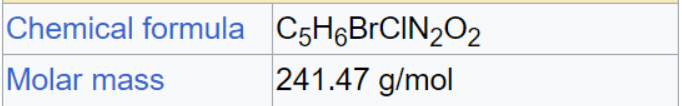
128.13/241.47 = 53.06% DMH.
If you use tabs, 20 lbs of tabs will add 10.612 lb of DMH, which is 63 ppm DMH and 50 ppm of sodium bromide.
To remove 10 ppm of DMH, you need to remove 3,175 gallons and add fresh water.
Molar mass: 285.923 g·mol−1
Formula: C5H6Br2N2O2
Symbol: Br. Atomic mass: 79.904 grams per mole.
79.904 x 4 = 319.616 (2Br2).
319.616/241.47 = 132.36% "Available Bromine" as Br2.
53.06% + 132.36% = 185.42%.
C5H6BrClN2O2 + 2H+ + 3Br- --> C5H8N2O2 + Cl- + 2Br2.
One mole of BCDMH provides 1 mole of DMH, 1 mole of bromide (after reduction) and 1 mole of chloride.
It also provides 2 moles of bromine (Br2) based on how bromine is reported.
2 moles of Br2 = 319.616 grams, which is why the percentage of available bromine is higher than 100% of the mass of BCDMH.
319.616/285.923 = 111%.
1 mole of DBDMH or 1 mole of BCDMH provides 2 moles of Br2.
20 lbs of tabs will add 26.47 lb of bromine, which is 158 ppm bromine.
Last edited:
From previous posts:
You should be diluting the water based on person hours. 1 Person-Hour is 1 person for 1 hour. Replace 8 gallons per person hour every day to keep the water fresh.
So... assuming a fresh fill, draining and adding 500 gallons per week might be sufficient to keep the water indefinitely assuming average Person-Hours of less than 9 per day (500 gallon replacement / 7 = 71.4 gallons per day / 8 = 8.9 Person-Hours per day. It would be interesting to monitor use of the indoor pool over a set period of time. I highly doubt that it exceeds 9 Person-Hours per day on average. We have a few regular swimmers that use it for maybe 1/2 hour a few days per week and occasionally a grandparent with a few grand kids for a couple hours.... maybe once or twice per week. I would definitely describe it as a "low use" pool. I have an email list of our regular pool users. I may just take a poll of their weekly use to see if I am in the ballpark on overall use of the pool.
You should be diluting the water based on person hours. 1 Person-Hour is 1 person for 1 hour. Replace 8 gallons per person hour every day to keep the water fresh.
So... assuming a fresh fill, draining and adding 500 gallons per week might be sufficient to keep the water indefinitely assuming average Person-Hours of less than 9 per day (500 gallon replacement / 7 = 71.4 gallons per day / 8 = 8.9 Person-Hours per day. It would be interesting to monitor use of the indoor pool over a set period of time. I highly doubt that it exceeds 9 Person-Hours per day on average. We have a few regular swimmers that use it for maybe 1/2 hour a few days per week and occasionally a grandparent with a few grand kids for a couple hours.... maybe once or twice per week. I would definitely describe it as a "low use" pool. I have an email list of our regular pool users. I may just take a poll of their weekly use to see if I am in the ballpark on overall use of the pool.

