Seems like we are going through much more muriatic acid this year after adding a liquid chlorine feeder and acid feeder to our outdoor pool. I understand that liquid chlorine causes a temporary rise in pH, but that it will fall back down as the chlorine gets used up (correct?) Is it possible that the pH probe on the Controller is causing a "too quick" reaction to the chlorine being fed into the pool? When I was manually dosing the pool with liquid chlorine, I was not using that much acid. I would see an initial rise in pH, but then it would settle back. Previously, it seems I was only going through a few gallons of acid in a season. Now, I put 4 gallons in the acid feeder and it is gone in about 2 weeks. Should I consider possibly eliminating the acid feeder and just manually adjust the pH? Also.... the pool company likes to keep TA around 100 ppm, but I had previously kept it around 70-80. Could that also be having an effect? On the Controller it says the recommended Alkalinity is 80-120 ppm.
Increased acid use?
- Thread starter JPMorgan
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Jun 24, 2021
- 15,980
- Pool Size
- 29000
- Surface
- Vinyl
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60 Plus
That is definitely the problem.Also.... the pool company likes to keep TA around 100 ppm, but I had previously kept it around 70-80. Could that also be having an effect?
What is the target pH?
Are you using ORP control?
7.5. Yes.... Intellichem controller uses ORP to feed the liquid chlorine. Is there another option?That is definitely the problem.
What is the target pH?
Are you using ORP control?
Do you recommend "pulling the plug" on the acid feeder? Could I just pull the tube out of the acid tank and start managing pH manually? We do use the acid tank for the indoor pool (bromine), but that doesn't seem to be a problem.That is definitely the problem.
What is the target pH?
Are you using ORP control?
One more bit of information.... before we switched to the liquid chlorine feeder, the pool was utilizing a trichlor feeder, but it was not utilizing an acid feeder. I wanted to get off the trichlor (due to the problems it causes with with CYA rise), but didn't want to manually dose the pool with liquid chlorine (did that for 2 years and got tired of doing that twice daily). Hence, the decision to go to the liquid chlorine feeder system.
- Aug 20, 2020
- 7,778
- Pool Size
- 27000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60
Trichlor is an acid and so using it means you need less acid to maintain pH. Now that its gone, you need the normal amount of acid. But the high TA and low pH target (7.5) will make you need more acid.One more bit of information.... before we switched to the liquid chlorine feeder, the pool was utilizing a trichlor feeder, but it was not utilizing an acid feeder. I wanted to get off the trichlor (due to the problems it causes with with CYA rise), but didn't want to manually dose the pool with liquid chlorine (did that for 2 years and got tired of doing that twice daily). Hence, the decision to go to the liquid chlorine feeder system.
Last edited:
Here is what is printed on the Controller as the recommended levels. Would you alter those? Where would you keep TA and pH? Right now I am maintaining at the high end of the recommended pH range at 7.5 and in the middle of the TA range at 100 ppm and am using more acid and baking soda than I ever did before.Trichlor is an acid and so using it means you need leas acid to maintain pH. Now that its gone, you need ghe normal amount of acid. But the high TA and low oH target (7.5) will make you need more acid.

wayner
LifeTime Supporter
- May 31, 2012
- 966
- Pool Size
- 100000
- Surface
- Vinyl
- Chlorine
- Salt Water Generator
- SWG Type
- Jandy Aquapure 1400
From the title of this thread I thought that this was going to be about what happened at Woodstock. 
A pH of 7.5 is going to require a lower TA.
I would keep the pH as high as you can legally keep it.
The CYA needs to be low for ORP control.
I would keep the TA at maybe 60 ppm.
I would keep the pH as high as you can legally keep it.
The CYA needs to be low for ORP control.
I would keep the TA at maybe 60 ppm.
A pH of 7.5 and a TA of 100 is impossible to maintain without going through tons of acid and tons of baking soda.
The acid lowers the TA and the baking soda raises the pH.
It's a losing battle.
If you reduce the TA and increase the pH, then there is less CO2 and less pH rise.
If you have to keep the TA over 60 and/or the pH below 7.8, then you might be better off using CO2 for pH control because CO2 reduces pH with no effect on TA.
The acid lowers the TA and the baking soda raises the pH.
It's a losing battle.
If you reduce the TA and increase the pH, then there is less CO2 and less pH rise.
If you have to keep the TA over 60 and/or the pH below 7.8, then you might be better off using CO2 for pH control because CO2 reduces pH with no effect on TA.
HCO3- + H+ --> H2CO3 --> H2O + CO2
pH..........%baking soda
6.35............50.00
6.5..............58.55
6.6..............64.01
6.7..............69.12
6.8..............73.81
6.9..............78.01
7.0..............81.71
7.1..............84.90
7.2..............87.62
7.35...........90.91
The Y axis is the percentage of baking soda that converts into carbon dioxide.
The X axis is the pH.

 www.wolframalpha.com
www.wolframalpha.com
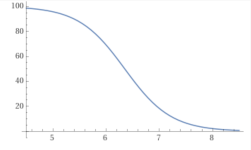
pH..........%baking soda
6.35............50.00
6.5..............58.55
6.6..............64.01
6.7..............69.12
6.8..............73.81
6.9..............78.01
7.0..............81.71
7.1..............84.90
7.2..............87.62
7.35...........90.91
The Y axis is the percentage of baking soda that converts into carbon dioxide.
The X axis is the pH.

y = 100-(100/(1+10^(6.35– x))), x from 4 to 8 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.

Ha!!!From the title of this thread I thought that this was going to be about what happened at Woodstock.
As you can see, the amount of CO2 at a pH of 7.5 is 3 times higher than at a pH of 8.0.
This means three times as much acid required and three times as much CO2 loss.
pH........% CO2
7.5..........6.6
7.6..........5.3
7.7..........4.3
7.8.........3.4
7.9.........2.7
8.0.........2.2
This means three times as much acid required and three times as much CO2 loss.
pH........% CO2
7.5..........6.6
7.6..........5.3
7.7..........4.3
7.8.........3.4
7.9.........2.7
8.0.........2.2
Is there a reason Pentair puts those recommended levels on their Controller? Seems like they want you to maintain a minimum TA of 80 and under 7.6 for pH. Would maintaining a TA of 60 and pH of 7.6 (or even 7.8) affect the Controller in any way? If I shoot for those levels would that likely cut down on the amount of acid and baking soda I'm going through?A pH of 7.5 and a TA of 100 is impossible to maintain without going through tons of acid and tons of baking soda.
The acid lowers the TA and the baking soda raises the pH.
It's a losing battle.
If you reduce the TA and increase the pH, then there is less CO2 and less pH rise.
If you have to keep the TA over 60 and/or the pH below 7.8, then you might be better off using CO2 for pH control because CO2 reduces pH with no effect on TA.
Here's what the health department regulations say about pH and Alkalinity levels:
- The pH of the pool water shall be maintained between 7.2 and 7.6.
- Alkalinity. The alkalinity of the pool water shall not be less than 50 nor more than 200 p.p.m. as calcium carbonate.
Note: in Pool Math, I get a CSI of 0.0 if I maintain these levels: FC 3.0 ppm; pH 7.6; TA 60 ppm; CYA 30 ppm CH 400 ppm Is this the ideal to shoot for?
If these changes fail to reduce chemical use, how complicated is installing a CO2 system? (The state makes us pay for architectural drawings almost every time we make a change in any equipment.) Are CO2 systems expensive... problematic?
That is what I would go for.shoot for 7.6 and 60?
I would use the highest FC allowable.If I try to maintain pH and Alkalinity at the levels noted above, what FC should I maintain?
Looks like a good target set.Note: in Pool Math, I get a CSI of 0.0 if I maintain these levels: FC 3.0 ppm; pH 7.6; TA 60 ppm; CYA 30 ppm CH 400 ppm Is this the ideal to shoot for?
Get some estimates.Are CO2 systems expensive... problematic?
Done correctly, they can be useful.
CO2 is rarely useful unless you are forced to maintain a TA that is too high and a pH that is too low.
Last edited:
Seems like they want you to maintain a minimum TA of 80 and under 7.6 for pH.
I cannot answer for the reasons that Pentair makes those recommendations.Is there a reason Pentair puts those recommended levels on their Controller?
Ask Pentair to explain their recommendations.
Affect how?Would maintaining a TA of 60 and pH of 7.6 (or even 7.8) affect the Controller in any way?
The pool industry in general is ignorant of the FC/CYA relationship and the role that CO2 plays in pH management.
The recommended levels are boilerplate recommendations from the 1950s.
Until the government officially changes the recommended levels, the industry is mostly stuck with recommendations that are less than ideal for everyone.

Last edited:
- Aug 20, 2020
- 7,778
- Pool Size
- 27000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60
It also says those are “recommended” but doesnt say they are “required”.
Thanks for the info. I'm going to try maintaining the higher pH and lower TA and monitor our chemical usage. Pool Math says target FC for CYA of 30 ppm is 2.0-6.0 ppm, so I will probably maintain at 4 ppm. The FC limit per regulations is 5 ppm. Will report back later on results.
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

