- Jul 29, 2018
- 785
- Pool Size
- 15000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-40
I've thought about this a long time. Researched lots of info on uses for the condensate from central air evaporators and dehumidifiers. No it's not suitable for drinking but is for watering plants OR for topping off your swimming pool. Check out for yourself the health and safety issues. Even the CDC is good with it. Here in the Gulf south (greater Houston area) it gets hot and it gets humid and mostly stays that way for a lot of the year. In addition to two A/C units--downstairs and up, I have a whole house Aprilaire dehumidifier that is independent of the two A/C systems (although it could be integrated if I had so opted--long story.) So three machines creating H2O condensate. So I finally did it--or rather the A/C guys did it. My downstairs AC and the dehumidifier, instead of going to the drain and the sanitary sewer, are draining into a rain barrel. My upstairs unit was too far away to easily and reliably connect to the pipe going outside. A faucet on the rain barrel connects a hose to the pool. It's a 40 gallon barrel, so I don't have to have the hose there all the time.
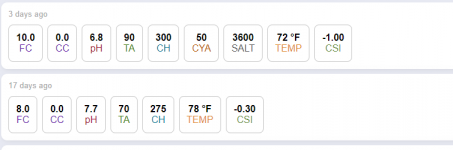
Right now I'm collecting first, then to the pool. We're in a fairly mild part of the year, but for the first five days, I've collected about 5 gallons of condensate per day. The pH of this water is 5.8 or so. Why, you ask, is it not 7.0? The process of air blowing over the evaporator coils as the water condenses causes carbon dioxide to be dissolved in the water. (Human skin has a pH of 5.5. Coffee 4.9, fruit juices range from 3.3 to 4.3.) I was hoping the low pH condensate would, in addition to compensating for evaporation, save having to add muriatic acid to the water. Not sure what 5 gallons a day will do for a 15,000 gallon pool. During warmer times of the year, according to local A/C people, I'll probably see 10 gallons a day or more. The AC guys said if both AC unit plus the dehumidifier were connected, I could get between 12 and 20 gallons a day in the summer.
My cheapskate friend asked, "How long will it take for savings on city water plus savings on muriatic acid to pay for the rerouting of condensate?" I don't expect a rapid payback or maybe never get my money back. I'm not an environmental nut, but I am happy to conserve when I can. Even with my current minimal 5 gallons a day, I'm saving 150 gallons a month. There are 10 million private swimming pools in the U.S. If only half are in a climate where they could do what I just did, that's 750 million gallons of water PER MONTH. 9 billion gallons of water per year. Free. Plus sum amount of muriatic acide savings. And that's based on my autumn savings of 5 gallons a day, which may double in the summer.
Right now I'm collecting first, then to the pool. We're in a fairly mild part of the year, but for the first five days, I've collected about 5 gallons of condensate per day. The pH of this water is 5.8 or so. Why, you ask, is it not 7.0? The process of air blowing over the evaporator coils as the water condenses causes carbon dioxide to be dissolved in the water. (Human skin has a pH of 5.5. Coffee 4.9, fruit juices range from 3.3 to 4.3.) I was hoping the low pH condensate would, in addition to compensating for evaporation, save having to add muriatic acid to the water. Not sure what 5 gallons a day will do for a 15,000 gallon pool. During warmer times of the year, according to local A/C people, I'll probably see 10 gallons a day or more. The AC guys said if both AC unit plus the dehumidifier were connected, I could get between 12 and 20 gallons a day in the summer.
My cheapskate friend asked, "How long will it take for savings on city water plus savings on muriatic acid to pay for the rerouting of condensate?" I don't expect a rapid payback or maybe never get my money back. I'm not an environmental nut, but I am happy to conserve when I can. Even with my current minimal 5 gallons a day, I'm saving 150 gallons a month. There are 10 million private swimming pools in the U.S. If only half are in a climate where they could do what I just did, that's 750 million gallons of water PER MONTH. 9 billion gallons of water per year. Free. Plus sum amount of muriatic acide savings. And that's based on my autumn savings of 5 gallons a day, which may double in the summer.