Wouldn't it be fun to string together 20 cells in series to make the system work?
Failing T-15 SWG Cell Experiment
- Thread starter mas985
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- May 3, 2007
- 18,071
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
Maybe if I had nothing better to do and owned a plumbing supply warehouse.
It's pretty simple.
Just make a manifold like this and install up to 24 cells.
You can use valves and valve actuators to open and close the parallel lines.
You can use relays that can power each cell individually as needed to maintain the desired amperage.
You need a power monitor and a logic circuit that can tell individual relays when to open and close and to tell the pump to increase speed to maintain 20 gpm per line.
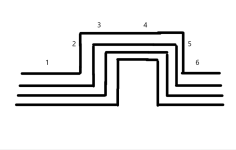
Just make a manifold like this and install up to 24 cells.
You can use valves and valve actuators to open and close the parallel lines.
You can use relays that can power each cell individually as needed to maintain the desired amperage.
You need a power monitor and a logic circuit that can tell individual relays when to open and close and to tell the pump to increase speed to maintain 20 gpm per line.

- May 3, 2007
- 18,071
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
- Jun 1, 2018
- 16,012
- Pool Size
- 26000
- Surface
- Vinyl
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
- May 3, 2007
- 18,071
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
[Month 10 - Cell#3]
The old T-15 cell running in T-5 mode has finally been replaced after about 10 months of operation in T-5 mode. At the time of replacement, it was operating at about 3.35A of current and salt readings from the unit were getting close to the shut down threshold of 2400 ppm so I wanted to replace the cell before it shut off to avoid operation without the SWG in order to assess production rates.
The operational age of the cell in T-15 mode was around 50 months so an additional 10 months of operation in T-5 mode increased the lifespan by 20% which is not too bad.
When I replaced the cell, current went from 3.35A up to 6.31A and a week later FC levels went from 7.5 ppm to 9 ppm even with a change in SWG setting from 90% for the old cell to 45% for the new cell with the same pump run time (24hr). With the old cell, FC/CYA was 12.5% while with the new cell, it FC/CYA now 15%. A couple of weeks before the transition, I intentionally targeted a higher FC/CYA than was really needed to make sure there was nothing growing in the water so I could better estimate the production rate change.
By my estimate the cell production rate ratio of old/new was about 0.42 and since the old/new cell current ratio was 0.53, the efficiency of the cell must have dropped by an additional 20% that cannot be explained by just the current alone. Probably additional side reactions that are favored with the plates losing some of the catalytic characteristics due to degradation.
The old T-15 cell running in T-5 mode has finally been replaced after about 10 months of operation in T-5 mode. At the time of replacement, it was operating at about 3.35A of current and salt readings from the unit were getting close to the shut down threshold of 2400 ppm so I wanted to replace the cell before it shut off to avoid operation without the SWG in order to assess production rates.
The operational age of the cell in T-15 mode was around 50 months so an additional 10 months of operation in T-5 mode increased the lifespan by 20% which is not too bad.
When I replaced the cell, current went from 3.35A up to 6.31A and a week later FC levels went from 7.5 ppm to 9 ppm even with a change in SWG setting from 90% for the old cell to 45% for the new cell with the same pump run time (24hr). With the old cell, FC/CYA was 12.5% while with the new cell, it FC/CYA now 15%. A couple of weeks before the transition, I intentionally targeted a higher FC/CYA than was really needed to make sure there was nothing growing in the water so I could better estimate the production rate change.
By my estimate the cell production rate ratio of old/new was about 0.42 and since the old/new cell current ratio was 0.53, the efficiency of the cell must have dropped by an additional 20% that cannot be explained by just the current alone. Probably additional side reactions that are favored with the plates losing some of the catalytic characteristics due to degradation.
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Probably additional side reactions that are favored with the plates losing some of the catalytic characteristics due to degradation.
The plates are titanium metal that is coated with ruthenium metal using a plasma discharge (physical vapor deposition). The plates will be thoroughly cleaned chemically prior to loading into the vacuum chamber and then undergo an RF sputter clean in an Ar discharge. This ensures that any oxide formed after cleaning is removed prior to the ruthenium being deposited. Ruthenium and titanium are reactive enough that there is no need for an adhesion layer like there is in some metal coating processes.
Once the ruthenium is gone, the titanium is exposed and it instantly anodizes in water to form a passive oxide coating. The oxide is still somewhat conductive but not nearly enough and so those area of the plate will simply produce hydrogen and oxygen by electrolysis of water. Chlorine gas formation will not be favored at all. One would expect more hydroxide formation in those areas which leads to high pH and calcium scale. Since the calcium scale will not adhere to the titanium oxide layer very well, it will spall off very easily. This is why you often see calcium snowflakes coming from returns as the SWG age.
- May 3, 2007
- 18,071
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
I did notice as time went on, that current would degrade rapidly and cleaning what scale that was on the plates did not help much. However, I found that soaking the cell in an acid solution 8:1 would improve the cell conductivity and boost the current for a short period of time. But overall, it seems consistent with what you are describing.Once the ruthenium is gone, the titanium is exposed and it instantly anodizes in water to form a passive oxide coating. The oxide is still somewhat conductive but not nearly enough and so those area of the plate will simply produce hydrogen and oxygen by electrolysis of water. Chlorine gas formation will not be favored at all. One would expect more hydroxide formation in those areas which leads to high pH and calcium scale. Since the calcium scale will not adhere to the titanium oxide layer very well, it will spall off very easily. This is why you often see calcium snowflakes coming from returns as the SWG age.
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
I did notice as time went on, that current would degrade rapidly and cleaning what scale that was on the plates did not help much. However, I found that soaking the cell in an acid solution 8:1 would improve the cell conductivity and boost the current for a short period of time. But overall, it seems consistent with what you are describing.
Titanium dioxide typically needs a very strong acid to dissolve it. In the lab, when we had to clean titanium hardware used in various plating baths, we would chemically etch the titanium using a mixture of peroxide and sulfuric acid, colloquially known as “piranha etch” (and believe me when I tell you that it feels like a piranha biting a chunk of flesh off when it splashes on you …). Gold deposits are first removed with Aqua Regia (3:1 molar ratio of hydrochloric and nitric acid mixture).
As soon as the titanium is removed from the acid, the fresh metal surface will passivate quickly in air. The conductivity of the oxide is a function of thickness and so what you saw with improved cell conductivity make sense as the oxide will continue to grow slowly and become more insulating.
At the anode, you will get mostly chlorine and maybe some oxygen.
At the cathode, you get production of hydrogen that is directly proportional to the production of chlorine gas or oxygen gas.
The process should be pH neutral either way.
Even if the anode only produced oxygen, the process would be pH neutral.
________________________
Water to produce hydrogen and oxygen.
6H2O→2H2+ O2 + 4OH-+ 4H+
Cathode 4H2O→ 2H2 + 4OH-.
Anode 2H2O→O2 + 4H+.
________________________
Water and chloride to produce hydrogen and chlorine gas.
Cathode 4H2O -> 2H2 + 4OH-.
Anode 4Cl- --> 2Cl2
________________________
2Cl2 +2H2O -> 3H+ + HOCl + OCl-.
HOCl + OCl- + uv light -> O2 + H+ + 2Cl-.
You produce the same amount of hydroxide whether the other product is oxygen or chlorine or a combination of both.
The ruthenium is a catalyst; if it gets compromised, the production of chlorine and/or oxygen will be reduced and the production of hydrogen will also be reduced by an equal amount.
The reaction at the cathode produces the same amount of hydrogen gas and hydroxide regardless of what is produced at the anode.
The production of oxygen at the anode results in acid (hydrogen ions) being produced at the same rate as hydroxide ions, which comes out pH neutral.
The production of chlorine at the anode results in acid (hydrogen ions) being produced at the same rate as hydroxide ions, which comes out pH neutral.
The cathode is exposed to hydroxide as hydrogen gas is produced and this will produce high pH and possibly scale.
If you are producing 50% oxygen, you have to run twice as long to get enough FC and this can increase the risk of scaling.
At the cathode, you get production of hydrogen that is directly proportional to the production of chlorine gas or oxygen gas.
The process should be pH neutral either way.
Even if the anode only produced oxygen, the process would be pH neutral.
________________________
Water to produce hydrogen and oxygen.
6H2O→2H2+ O2 + 4OH-+ 4H+
Cathode 4H2O→ 2H2 + 4OH-.
Anode 2H2O→O2 + 4H+.
________________________
Water and chloride to produce hydrogen and chlorine gas.
Cathode 4H2O -> 2H2 + 4OH-.
Anode 4Cl- --> 2Cl2
________________________
2Cl2 +2H2O -> 3H+ + HOCl + OCl-.
HOCl + OCl- + uv light -> O2 + H+ + 2Cl-.
You produce the same amount of hydroxide whether the other product is oxygen or chlorine or a combination of both.
The ruthenium is a catalyst; if it gets compromised, the production of chlorine and/or oxygen will be reduced and the production of hydrogen will also be reduced by an equal amount.
The reaction at the cathode produces the same amount of hydrogen gas and hydroxide regardless of what is produced at the anode.
The production of oxygen at the anode results in acid (hydrogen ions) being produced at the same rate as hydroxide ions, which comes out pH neutral.
The production of chlorine at the anode results in acid (hydrogen ions) being produced at the same rate as hydroxide ions, which comes out pH neutral.
The cathode is exposed to hydroxide as hydrogen gas is produced and this will produce high pH and possibly scale.
If you are producing 50% oxygen, you have to run twice as long to get enough FC and this can increase the risk of scaling.
Last edited:
- May 3, 2007
- 18,071
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
I believe the side reactions may be much higher than what one might expect. If you run the numbers for a T-15 cell, the published and measured new cell production rates are almost half of the ideal calculated production rate based upon the cell current alone.At the anode, you will get mostly chlorine and maybe some oxygen.

So based upon this data, there must be other significant reactions beside CL that consume electrons. Also, it looks like the published production rate is close to the 5.5 amp measurements which is interesting to note.
Only after mixing, and for the CL case after UV exposure, is the PH neutral. Near the electrodes, I think there is the potential for very high and low PH.At the cathode, you get production of hydrogen that is directly proportional to the production of chlorine gas or oxygen gas.
The process should be pH neutral either way.
Given the flow near the electrodes is laminar and goes to zero at the plate wall, there may not be a lot of turbulent flow mixing going on within the cell so the ions produced at one electrode move toward the opposite electrode mostly due to ionic mobility which is a fairly slow process.
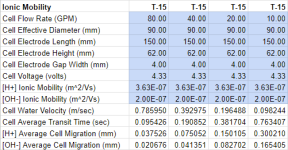
So there may not be much mixing going on before the end the of cell which means that the PH could be quite extreme at the two electrodes. But it also could mean that for bipolar plates, when one plate gets scaled due to high PH in one cycle, in the next cycle, some of the scale dissolves due to low PH although probably not in equal amounts. Also, this would be dependent on a rapid dissolution rate for the reaction
2Cl2 +2H2O -> 3H+ + HOCl + OCl-
If most of the CL2 dissolves after exiting the cell, then the PH near the anode would be closer to normal and would not offset the scale generated in the last cycle when it was operating as a cathode.
But even if that reaction occurs within the cell and the production products fully mix all within the cell, there is still a PH increase in the cell due to 4[OH-] vs 3[H+].
Reality is probably somewhere between these two extremes.
I have experienced cell scale even with a negative CSI for the main pool water. I believe this has to do with the higher PH near the cathode.The cathode is exposed to hydroxide as hydrogen gas is produced and this will produce high pH and possibly scale.
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Borates would help greatly in this situation. They have their highest buffering capacity right around a pH of 9.2. With a high enough concentration (I prefer to run my borate levels 80-100ppm, although I am a bit low now at 56ppm), the pH rise will be clamped by the boric acid/borate equilibrium. Theoretically this should keep the cell cleaner and lead to less pH rise. On the downside, when adjusting pH in the pool water, it takes more acid to move the needle. But that’s really a minimal cost on my case.
I suspect that oxygen is the primary byproduct.
You might get some current going through the water.
It would be interesting if they could report the amount of hydrogen gas produced.
The amount of hydrogen will probably be close to the theoretical amount.
You might get some current going through the water.
It would be interesting if they could report the amount of hydrogen gas produced.
The amount of hydrogen will probably be close to the theoretical amount.
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
These are bipolar cells and so there is no physical compartment between the anode and cathode. All of the chemical species produced simply mix into the the water stream aside from the hydrogen gas. I suppose if there was a good plumbing setup where you could dissolve all the chlorine and capture all the hydrogen, you might be able to measure output … until someone decides to light up their cigarette near the setup and then next thing you know your -


- May 3, 2007
- 18,071
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
Been there, done that. When I first built the pool, I collected enough hydrogen gas for a fairly impressive woosh!
- May 23, 2015
- 25,695
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Given the flow near the electrodes is laminar and goes to zero at the plate wall, there may not be a lot of turbulent flow mixing going on within the cell so the ions produced at one electrode move toward the opposite electrode mostly due to ionic mobility which is a fairly slow process.
Just my thoughts but I actually wonder how true this is. The anodes are loose plates, except for the end plates and center plate, and are held in place by a plastic cage. Given the sharp edges, I would think the flow between the plates is probably mixed at best. There’s a certain amount of electric field and current bulging that takes place at the ends of the plates due to flow. Thus I think the current distribution is a lot less uniform and non-ideal. There’s probably enough variations in cell designs out there in different products but I doubt anyone designs these cells to that level of detail.
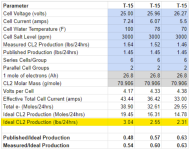
One ampere is equal to 1 coulomb (C) moving past a point per second.
43.44 amps for 24 hours = 3,753,216 Coulombs.
3,753,216 Coulombs = 38.9 Faraday = 38.9 Moles of electrons.
1 Mole of electrons produces 0.5 moles of chlorine gas.
Ideal is 19.45 moles of chlorine gas at 70.906 grams per mole, which is 1.379 kilograms or 3.04 lb of chlorine gas.
Measured Cl2 is 1.64 lbs, which is 54% of ideal.
21 moles of electrons were used for the production of chlorine gas, which leaves 17.9 moles of electrons unaccounted for.
1 Mole of electrons produces 0.25 moles of oxygen gas.
If oxygen is the other product, then you should get 4.475 moles of oxygen gas at 32 grams per mole = 143.2 grams = 0.3157 lb oxygen gas.
1 Mole of electrons produces 0.5 moles of hydrogen gas.
38.9 Moles of electrons should produce 19.45 moles of hydrogen at 1 gram per mole = 19.45 grams of hydrogen gas.
43.44 amps for 24 hours = 3,753,216 Coulombs.
3,753,216 Coulombs = 38.9 Faraday = 38.9 Moles of electrons.
1 Mole of electrons produces 0.5 moles of chlorine gas.
Ideal is 19.45 moles of chlorine gas at 70.906 grams per mole, which is 1.379 kilograms or 3.04 lb of chlorine gas.
Measured Cl2 is 1.64 lbs, which is 54% of ideal.
21 moles of electrons were used for the production of chlorine gas, which leaves 17.9 moles of electrons unaccounted for.
1 Mole of electrons produces 0.25 moles of oxygen gas.
If oxygen is the other product, then you should get 4.475 moles of oxygen gas at 32 grams per mole = 143.2 grams = 0.3157 lb oxygen gas.
1 Mole of electrons produces 0.5 moles of hydrogen gas.
38.9 Moles of electrons should produce 19.45 moles of hydrogen at 1 gram per mole = 19.45 grams of hydrogen gas.
Water to produce hydrogen and oxygen.
6H2O→2H2+ O2 + 4OH-+ 4H+
Cathode 4H2O→ 2H2 + 4OH-.
Anode 2H2O→O2 + 4H+.
________________________
Water and chloride to produce hydrogen and chlorine gas.
Cathode 4H2O -> 2H2 + 4OH-.
Anode 4Cl- --> 2Cl2
________________________
The production of hydrogen happens at the cathode whether the anode is producing chlorine or oxygen gas.
This leaves behind hydroxide, which is going to always have a risk of producing scale.
Lowering the carbonate levels and using borate can help.
Reversing polarity helps.
The chlorine gas is acidic, which can help prevent pH rise.
The production of oxygen gas leaves behind hydrogen ions, which lowers pH.
Besides the production of oxygen the only other reaction might be heat from current traveling through the water.
To heat water, you need AC current.
Using DC current causes charges to build up on the electrodes and this reduces the ability for current to flow.
For AC, you mostly get heat and some electrolysis.
For DC you get mostly electrolysis and some heat from current flowing through the water.
Heat energy is I2R, which is current squared x resistance of the water.
P=I^2R equation, also known as Joule's Law.
If you can measure the amount of hydrogen gas produced and the amount of heat produced, then you should be able to account for almost all of the electrons used.
6H2O→2H2+ O2 + 4OH-+ 4H+
Cathode 4H2O→ 2H2 + 4OH-.
Anode 2H2O→O2 + 4H+.
________________________
Water and chloride to produce hydrogen and chlorine gas.
Cathode 4H2O -> 2H2 + 4OH-.
Anode 4Cl- --> 2Cl2
________________________
The production of hydrogen happens at the cathode whether the anode is producing chlorine or oxygen gas.
This leaves behind hydroxide, which is going to always have a risk of producing scale.
Lowering the carbonate levels and using borate can help.
Reversing polarity helps.
The chlorine gas is acidic, which can help prevent pH rise.
The production of oxygen gas leaves behind hydrogen ions, which lowers pH.
Besides the production of oxygen the only other reaction might be heat from current traveling through the water.
To heat water, you need AC current.
Using DC current causes charges to build up on the electrodes and this reduces the ability for current to flow.
For AC, you mostly get heat and some electrolysis.
For DC you get mostly electrolysis and some heat from current flowing through the water.
Heat energy is I2R, which is current squared x resistance of the water.
P=I^2R equation, also known as Joule's Law.
If you can measure the amount of hydrogen gas produced and the amount of heat produced, then you should be able to account for almost all of the electrons used.
Hayward, Pentair and Jandy are providing conservative production numbers based on actual measured results.
Other companies are publishing numbers closer to the Ideal Production.
It is important to realize that some companies production numbers are not realistic.
For example, the Universal 55 and the RJ 60 Plus will probably not produce the amount indicated.
They also allow the salinity to go as high as 4,500 ppm.
So, maybe at a water temperature of 104 degrees and a salinity of 4,500 ppm, they might get somewhere near the published production numbers.
Assuming the same efficiency, the amperage required to get to 3 lbs of production would need to be about 13.24 amps.
This seems very unlikely.
At 10 amps, you might get to 2.25 lbs of chlorine gas per day.
The manuals show 7.8 amps and the high salt probably comes on at 8 amps like the Aquarite.
At 8.0 amps, the production might get to about 1.8 lbs.
That is probably the limit for most SWGs.
The IC-60 shows 2.00 lbs, which is about 8.8 amps.
The fuse is 10 amps, so that is cutting it close.




Other companies are publishing numbers closer to the Ideal Production.
It is important to realize that some companies production numbers are not realistic.
For example, the Universal 55 and the RJ 60 Plus will probably not produce the amount indicated.
They also allow the salinity to go as high as 4,500 ppm.
So, maybe at a water temperature of 104 degrees and a salinity of 4,500 ppm, they might get somewhere near the published production numbers.
Assuming the same efficiency, the amperage required to get to 3 lbs of production would need to be about 13.24 amps.
This seems very unlikely.
At 10 amps, you might get to 2.25 lbs of chlorine gas per day.
The manuals show 7.8 amps and the high salt probably comes on at 8 amps like the Aquarite.
At 8.0 amps, the production might get to about 1.8 lbs.
That is probably the limit for most SWGs.
The IC-60 shows 2.00 lbs, which is about 8.8 amps.
The fuse is 10 amps, so that is cutting it close.





Attachments
Last edited:
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.